MCDB 310 Macro Molecules
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
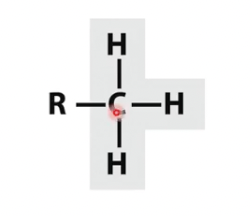
Methyl
C and H only
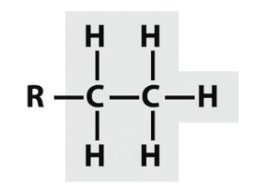
Ethyl
C and H only
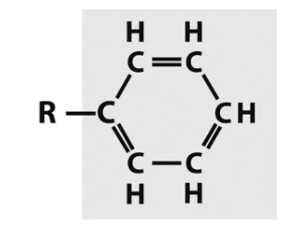
Phenyl
C and H only
Aromatic
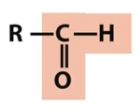
Carbonyl
aldehyde
oxygen containing (note titratable groups)
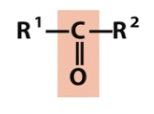
Carbonyl
ketone
oxygen containing groups (note titratable groups)
participates in Keto-Enol tautomerization
Keto-Enol Tautomerization
A chemical equilibrium between a keto form (a ketone or an aldehyde) and an enol in organic chemistry (alcohol). Tautomers are claimed to exist between the keto and enol forms
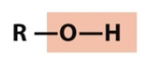
Hydroxyl
alcohol
oxygen containing groups (note titratable groups)
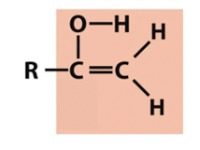
Enol
participates in keto enol tautomerization
oxygen containing groups (note titratable groups)
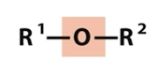
Ether
oxygen containing groups (note titratable groups)
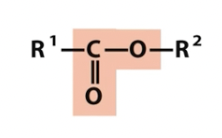
Ester
oxygen containing groups (note titratable groups)
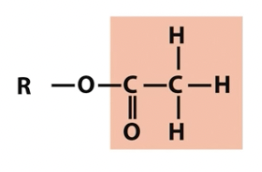
Acetyl
oxygen containing groups (note titratable groups)
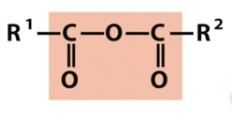
Anhydride
2 carboxylic acids
very high energy
oxygen containing groups (note titratable groups)
unfavorable to form; favorable to break
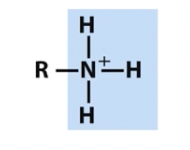
Amino
protonated
nitrogen containing groups (note titratable positions and unpaired electrons)
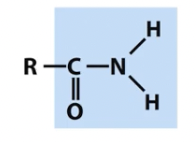
Amido
nitrogen containing groups (note titratable positions and unpaired electrons)
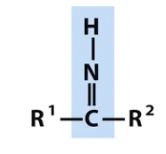
Imine
nitrogen containing groups (note titratable positions and unpaired electrons)
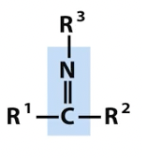
N-Substituted imine
Schiff base
nitrogen containing groups (note titratable positions and unpaired electrons)
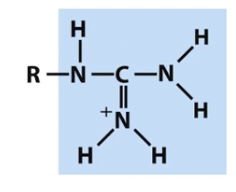
Guanidinium
nitrogen containing groups (note titratable positions and unpaired electrons)
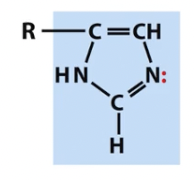
Imidazole
nitrogen containing groups (note titratable positions and unpaired electrons)
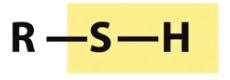
Sulfhydryl
Reduced
Sulfhydryls and disulfide can interchange in reversible reaction
Condensation of sulfhydryl and carboxylate forms a thioester (NOT an oxygen ester)
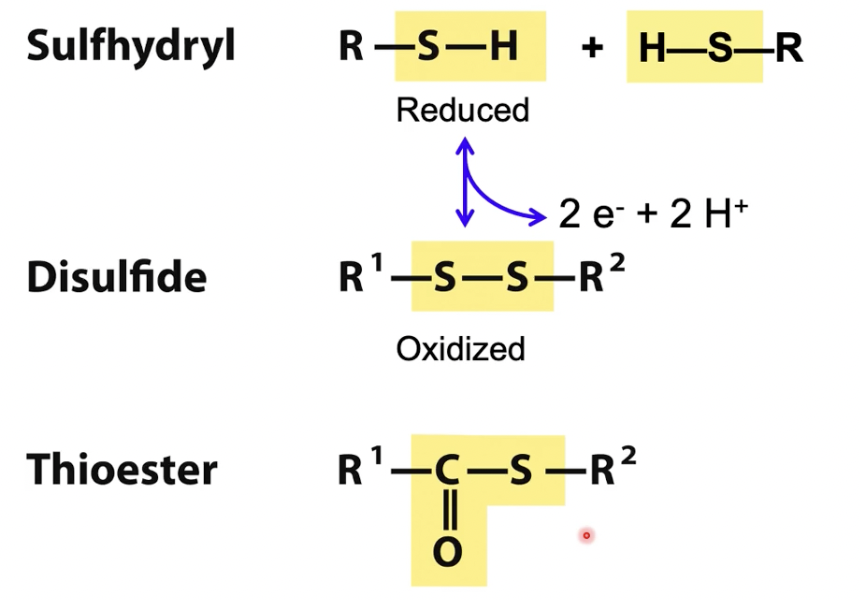

Disulfide
oxidized
Sulfhydryls and disulfide can interchange in reversible reaction
Condensation of sulfhydryl and carboxylate forms a thioester (NOT an oxygen ester)
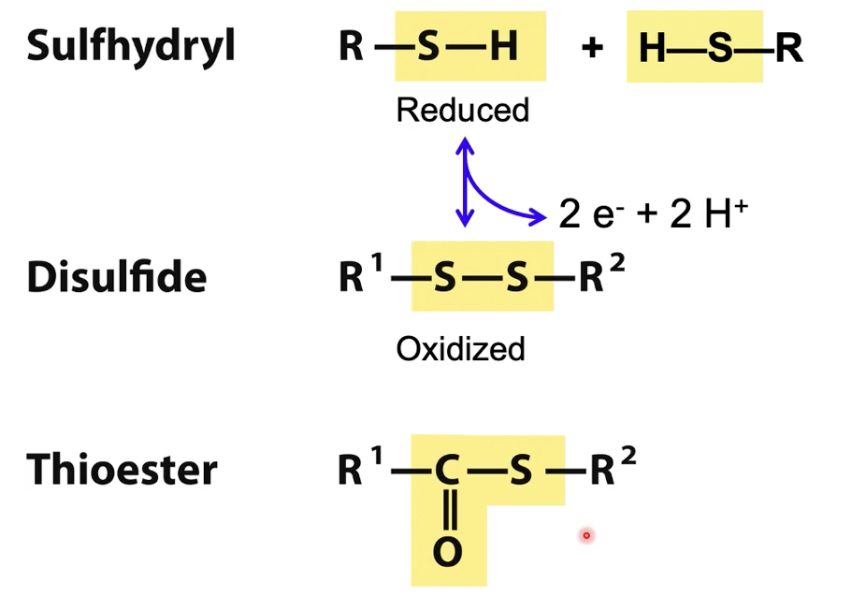
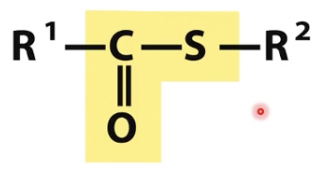
Thioester
condensation of sulfhydryl and carboxylate forms a thioester
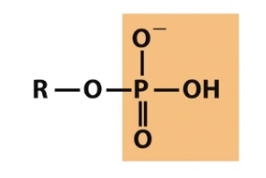
Phosphoryl
phosphoryl is NOT phosphate
note multiple titratable positions on phosphoryl group
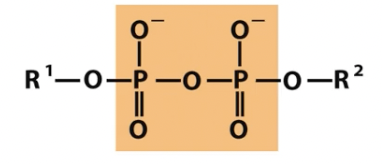
Phosphoanhydride
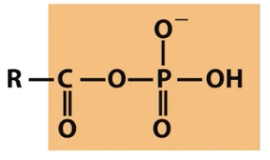
Mixed anhydride
carboxylic acid and phosphoric acid
also called acyl phosphate
How many functional groups to biological molecules typically have?
several