Lecture 2: Hormones Receptors and Rhythms
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
55 Terms
what does the fate of a cell depend on
depends on a multitude of extracellular signals
what are the different responses of cells to signals?
survive
divide
differentiate
what happens if a cell receives NO signals
apoptosis
explain when signal binds to receptor
the receptor will recognize the signal
change in intracellular network of proteins
activate of target gene/ protein
cellular response
what are examples of possible target proteins in a cell
metabolic enzyme—> altered metabolism
gene regulatory protein—> altered gene expression
cytoskeletal protein—> alter shape or movement
what is the function of signal mediators?
amplify signal during transmission
what is the definition of cellular response?
cell that has been stimulated by process of cell transduction
explain the whole transmission/transduction pathway
look at drawimg
what are the two different classes of signal receptors
Based on THEIR LOCATIONS
cell surface receptors
intracellular receptors
explain cell surface receptors
hydrophilic signal molecule binds to cell surface receptor
explain intracellular receptors
small hydrophobic signal molecule—> need a carrier protein
intracellular found in nucleus receptor
explain receptor types of hydrophilic vs hydrophobic hormones
hydrophilic—> cell surface
hydrophobic—> intracellular
what are examples of hormones that have cell surface receptors
prostaglandins and leukotrienes—> BOTH are hydrophobic which is an expection to the general trend
what are the receptors for steroids and bile acids
cell surface and intracellular
can chemical signals be nutrients/metabolites? + how can they be signalling molecules
YES ---> can be signalling molecules as they have receptors
what are the different metabolites that can act as signalling molecules?
1. lactate
2. ketone bodies (BHB)
3. succinate
4. alpha - ketoglutarate
5. fatty acids
6. calcium
7. bile acids
what are the 3 main domains of a cell surface receptor and their locations
ectodomain
hydrophobic transmembrane domain
cytoplasmic domain
what does a signal first bind to
ectodomain
explain the structure of the ectodomain
rich with cysteine residues—> this will have S-S bonds for folding and is needed for conformational changes
also is glycosylated—→ AA are attached to carbohydrate moieties which signals can attach to
do free ectodomains have functions and with example
YES! they can circulate as hormone-binding proteins
EX: the GH receptor's ectodomain acts as a GH binding protein - serves as a carrier protein
what happens if ectodomain is cleaved?
can cause endocrine disease
what is the role of the cytoplasmic domain
relays the signal by inducing a signaling cascade ( relay of conformational chnages of signaling proteins)
what are conformational changes induced by in cytoplasmic domain
phosphorylation of proteins
binding between proteins
how are signaling proteins mainly modulated by
phosphorylation
what amino acids are most commonly phosphorylated
Serine
threonine
tyrosine
—> all have free OH group that can phosphorylated by KINASES
explain how phosphorylation happens
look at notes
P1—> activated P1 by protein kinases
P1 can then activate P2
remove phosphate by protein phosphatase
what enzymes can reverse phosphorylation
phosphatase
what are the 2 things that are needed to power phosphorylation?
1. protein kinase
2. ATP ---> where the phosphate comes from
how does each phosphorylation step allow for signal amplification?
there is a lag period between being activated + deactivated that allows for signal amplification
which aa are the commonly phosphorylated?
serine and threonine more abundant than tyrosine
when does tyrosine phosphorylation usually occur?
occurs at the beginning of a the cascade bc many first receptors have induce tyrosine kinase activity
what does the phosphorylated tyrosine serve as
serve as a docking site for downstream signal proteins
means that it creates a structure that allows for other proteins to come + sit
what amino acid sequence mediates the docking to phosphorylated tyrosine?
SH2 and SH3 domains is conserved and diagnostic for proteins involved in the signaling cascade
what are the types of cell surface receptors
GPCR
RTK—> intrinsic and recruited
Serine-threonine kinase receptors ( RSTK)
Explain the GPCR pathway
the first messenger ( SIGNAL) will bind to receptor
binding will activate G protein and the alpha subunit will be shuttled to activate adenylyl cyclase
adenylyl cyclase will convert ATP to cAMP
cAMP will activate PKa
PKa will phosphorylate inactive protein—> will become active
active protein can bring about cellular response
what does adenylyl cyclase do?
ATP to cAMP
what is the structure of PKA
exists as a dimer
composed of: PKA c, PKA r, AKAP
what is PKA c
catalyze phosphorylation
what is PKA R
regulates PKA
how many cAMP is needed to activate PKA complex
4 cAMP—> means for 4 ATPs
explain PKA activation
cAMP binds to the regulatory subunits of PKA.
The catalytic subunits are released.
The catalytic subunits phosphorylate target proteins.
what cellular proteins are regulated by cAMP and PKA
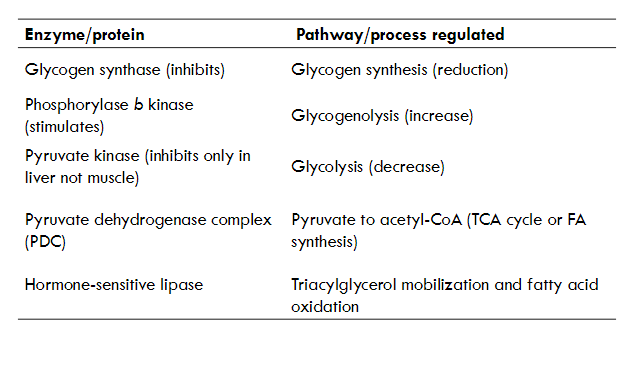
what is the second activation of target of GPCR and IP3
phospholipase C
Explain GPCR signaling through IP3 and Ca
signal binds to receptor
G protein is activated
alpha subunit shuttles and activates phospholipase C
phospholipase C converts PIP2 to IP3 and DAG
IP3 mobilizes intracellular Ca2+
Ca2+ acts as SECOND MESSENGER
Ca2+ activates calmodulin
calmodulin complex activates CaM kinase
CaM kinase activates target protein
cellular response
what does phospholipase C do
converts PIP2—> IP3 and DAG
what is the fate of DAG
DAG activates PKC
acts on specific protein
cellular response
what is the pathway of signal control desensitization-resensitization cycle of GPCR?
epinephrine binds to beta-adrenergic receptor triggers dissociation of Gsby from Gsalpha
Gsby recruits bARK to membrane where it phosphorylates Ser residues at carboxyl terminus of receptor
b-arrestin (barr) binds to phosphorylated carboxyl terminal domain of receptor
receptor arresetin complex enters the cell by endocytosis
dissociates and returns to cell surface
what experiment did they perform to learn that for BARR to be recruited NEED a cytoplasmic domain?
had 2 cells ---> 1 w normal cytoplasmic domain thath allowed Barr to b recruited and 1 w/o cytoplasmic domain
in the TRH receptor added rhodamine fluorescent molecule to be able to see it and added green fluorescent protein to BARR
SAW: first cells that had a cytoplasmic domain glowed bc of the florescent dye on BARR
- second cells did not light up
what are five ways target cells can become desensitized to a signal molecule?
1. receptor sequestration ---> moved away from the surface + recycled back
2. receptor down - regulation ---> once internalized it is degraded
3. receptor inactivation
4. inactivation of signalling protein
5. production of inhibitory protein ---> negative regulator is induced
What does Gs alpha do?
activates adenylate cyclase
S = stimualtory
what does Gi alpha do?
inhibits adenylate cyclase
I = inhibitory
what is the role of Gq alpha?
activates phospholipase C
what is the role of Go alpha?
activates ion channels
what is the role of G12/13 alpha?
regulates actin cytoskeleton
can hormones use more than one g protein
YES
the receptors have specfic alpha subunit that will have the specific response