Handout 1A: Amino Acids - Electronics and Acid-Base Chemistry
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
Amino Acids: Overall Structure
a.a’s are difunctional compounds that contain both an amine and a carboxylic acid functionality
acid has priority in naming
carbons b/w the functional groups designated ⍺, β, γ, δ, etc. starting from the carbonyl
20 common a.a’s found in proteins (all ⍺-amino acids)
differ in R group/side chain
Chirality
glycine is achiral
other 19 common amino acids are chiral; each contains a stereogenic center at the ⍺-carbon
all are in “L” configuration; when drawn in Fischer Projection w/ the most oxidized carbon (CO2H) at the top, R group at the botoom, the NH2 is on the left
threonine and isoleucine have a second stereogenic center within their side chains
Fischer Projections
vertical lines go into the page (dashes)
horizontal lines come out the page (wedges)
a switch of the two substituents leads to the opposite configuration (R → S, S → R, changes enantiomer)
two switches and you are back where you started
Amino Acids: R vs S Configuration
the ⍺-carbon of cysteine has R configuration
other 18 common acids have S configuration
Acid-Base Properties of Amino Acids
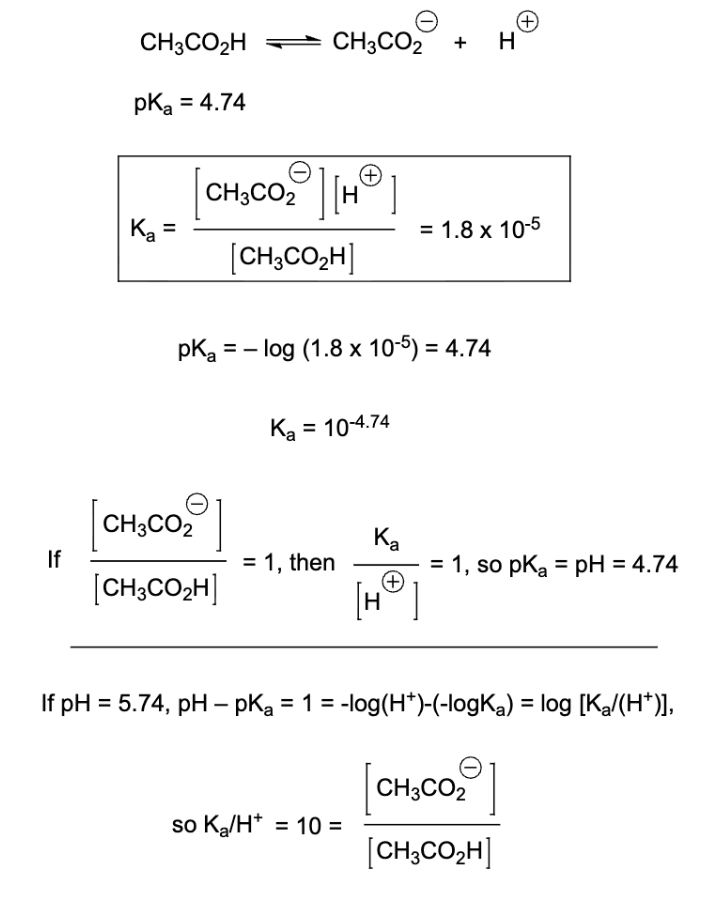
when CB/CA = 1, pKa = pH
when pH < pKa, CA dominates
when pH > pKa, CB dominates
when the pH is one pH unit above the pKa, there is 10x more CB present than CA
pKa, Ka Definitions
pKa: indicates how readily an acid donates a proton
lower pKa = stronger acid
Ka: acid dissociation constant
larger Ka = stronger acid
smaller Ka = weaker acid
Simpel Amino Acids
side chain is neither acidic nor basic
exist as zwitterions in neutral aqueous solution (contain both + and - charges)
pKa carboxylic acid = 2.3
pKa ammonium = 9.6
Why is the pKa of an a.a lower than acetic acid (4.74)
the amino group makes the carboxyl group more acidic by stabilizing its CB (zwitterionic state)
NH3+ pulls electron density away from COO- (spreads out - charge)
electronically stabilizing
inductive effect
resonance and sterics do not come into play
Factors that might affect a reaction
sterics: physical crowding around reactive site can block reactants from approaching each other
electronics: distribution of electron density in a molecule, EDG or EWG can stabilize/destabilize charges or T.S
hydrogen bonding: formation of H-bonds can stabilize reactants, intermediates or T.S (changes reactivity or orientation of molecules)
solvation: refers to interactions b/w reactants and solvents; solvents can stabilize charged species, influence nucleophilicity/electrophilicity
Within Electronics: Induction
induction: electron density is shifted thru σ bonds due to electronegativity differences
EWG stabilize (-) charge and destabilize (+) charge, EDG do the opposite
Within Electronics: Resonance
resonance (delocalization of charge): e- density is delocalized over multiple atoms via π system or lone pairs
delocalization stabilizes charged intermediates and T.S, lowers activation energy, favors resonance-stabilized products
Within Electronics: Hybridization
hybridization: s-character of an orbital affects electron holding ability
more s-character (sp>sp2> sp3) hold electrons more tightly
Within Electronics: Octets
octets (atoms like to have full octets): more stable
Within Electronics: Aromaticity
aromaticity (achieving aromaticity accords stabilization): very stabilized by cyclic, conjugated π electron delocalization
rxs that form/preserve aromaticity are favored
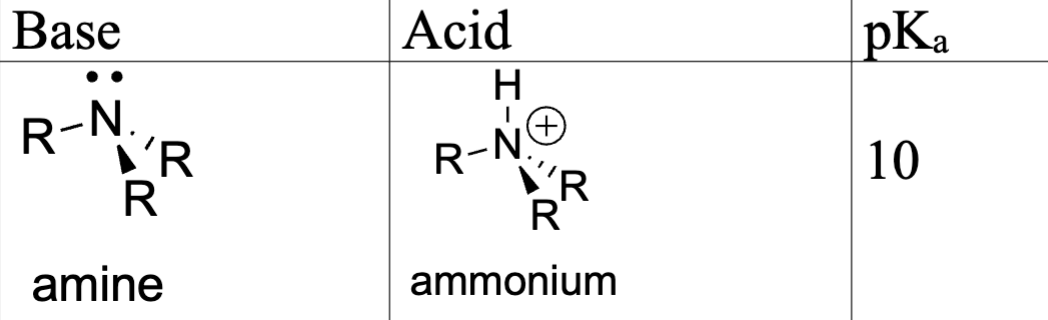
Compare amine, pyridine, and pyrrole basicity: Amine
N hybridization: sp3 (more p-character; e-’s are further from the nucleus; more stable)
(+) charges held closer to the nucleus are less stable; (-) charged have opposite effect
pKa = 10
conjugate acid: ammonium
lone pair = sp3 orbital (l.p is fully available, most basic)
Compare amine, pyridine, and pyrrole basicity: Pyridine
N hybridization: sp2
e- are held closer to nucleus
lone pair: sp2 orbital (not part of aromatic sextet)
conjugate acid: pyridinium
wants to get rid of proton more than ammonium
more acidic than ammonium due to hybridization
pKa = 5
aromatic (has resonance structures)
Key idea: Lone pair is available but stabilized by higher s-character → less basic than amines
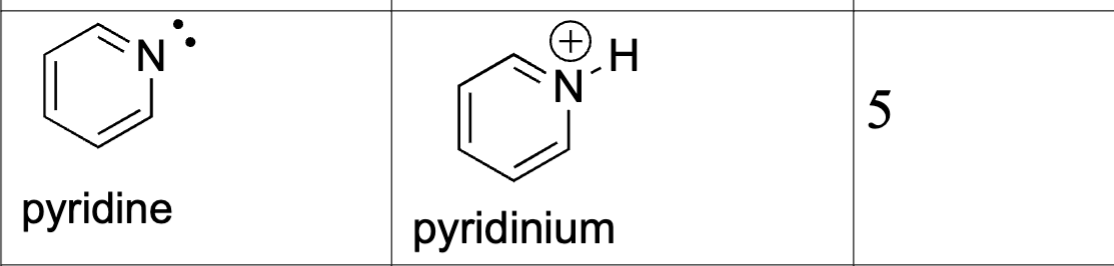
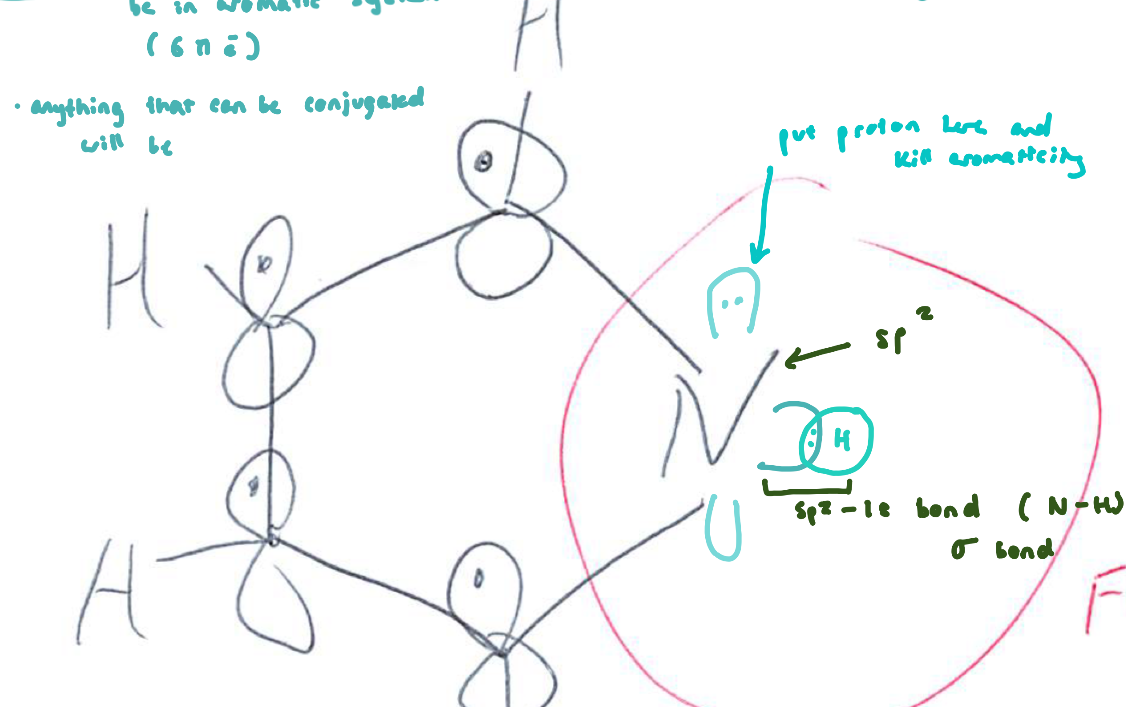
Compare amine, pyridine, and pyrrole basicity: Pyrrole
N hybridization: sp²
Lone pair: p orbital, part of aromatic sextet
Conjugate acid: pyrrolium
pKa (acid): ~0 (very weak base)
Key idea: Lone pair needed for aromaticity → least basic
Why is sp2 l.p held more tightly?
an sp2 l.p is held more tightly to the nucleus and thus has less affinity for a proton
the more s-character, the closer the orbital/electrons/charge are to the nucleus
this is stabilizing for a (-) charge, but destabilizing for a (+) charge
eg. the l.p og pyridine’s l.p is more stable, less basic than the l.p of an amine
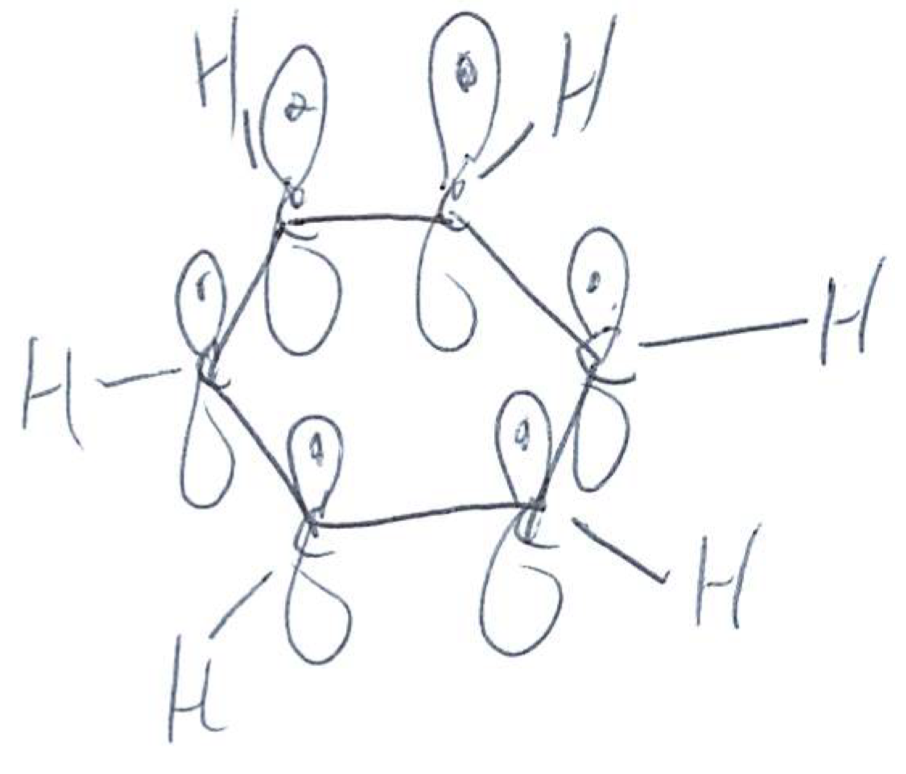
Compare benzene, pyridine, and pyrrole: Benzene
Atoms in ring: 6 carbons
Aromatic? Yes (6 π electrons)
all double bands are conjugated
Lone pairs? None
each C is sp2 hybridized
Basicity: Essentially non-basic
Key idea: Pure π system, no heteroatom to protonate
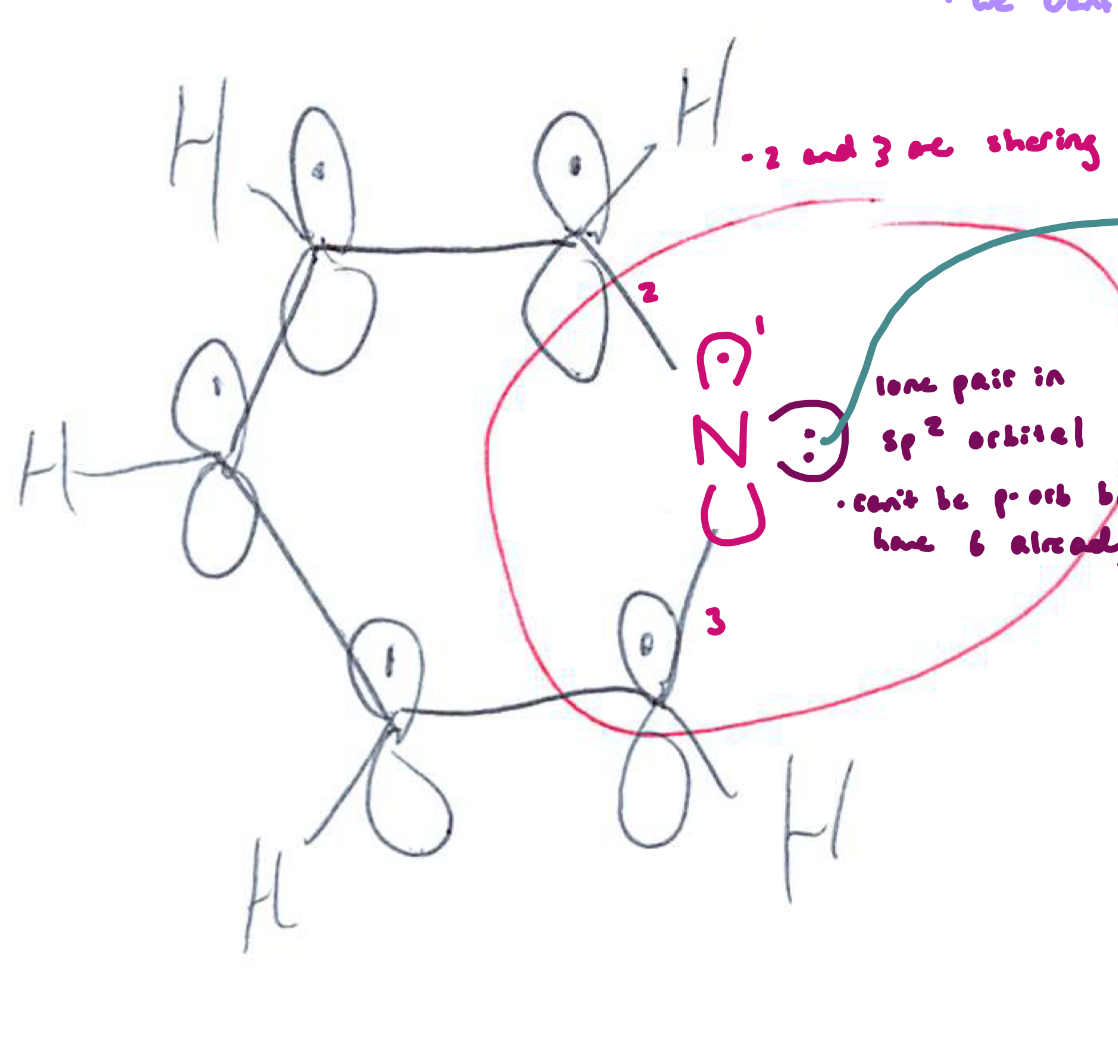
Compare benzene, pyridine, and pyrrole: Pyridine
Atoms in ring: 5 carbons + 1 nitrogen
N hybridization: sp²
Aromatic? Yes (6 π electrons)
Lone pair: sp² orbital, not part of aromatic sextet
Basicity: Weak base (pKa of conjugate acid ≈ 5)
Key idea: Lone pair is available → can be protonated without breaking aromaticity
Compare benzene, pyridine, and pyrrole: Pyrrole
Atoms in ring: 4 carbons + 1 nitrogen
N hybridization: sp²
Aromatic? Yes (6 π electrons)
Lone pair: p orbital, part of aromatic sextet
p-orbitals are higher energy than s-orbitals (by promoting to p-orbitals → get conjugation)
Basicity: Very weak base
Key idea: Protonation destroys aromaticity → strongly disfavored
Pyrrole + charge delocalization
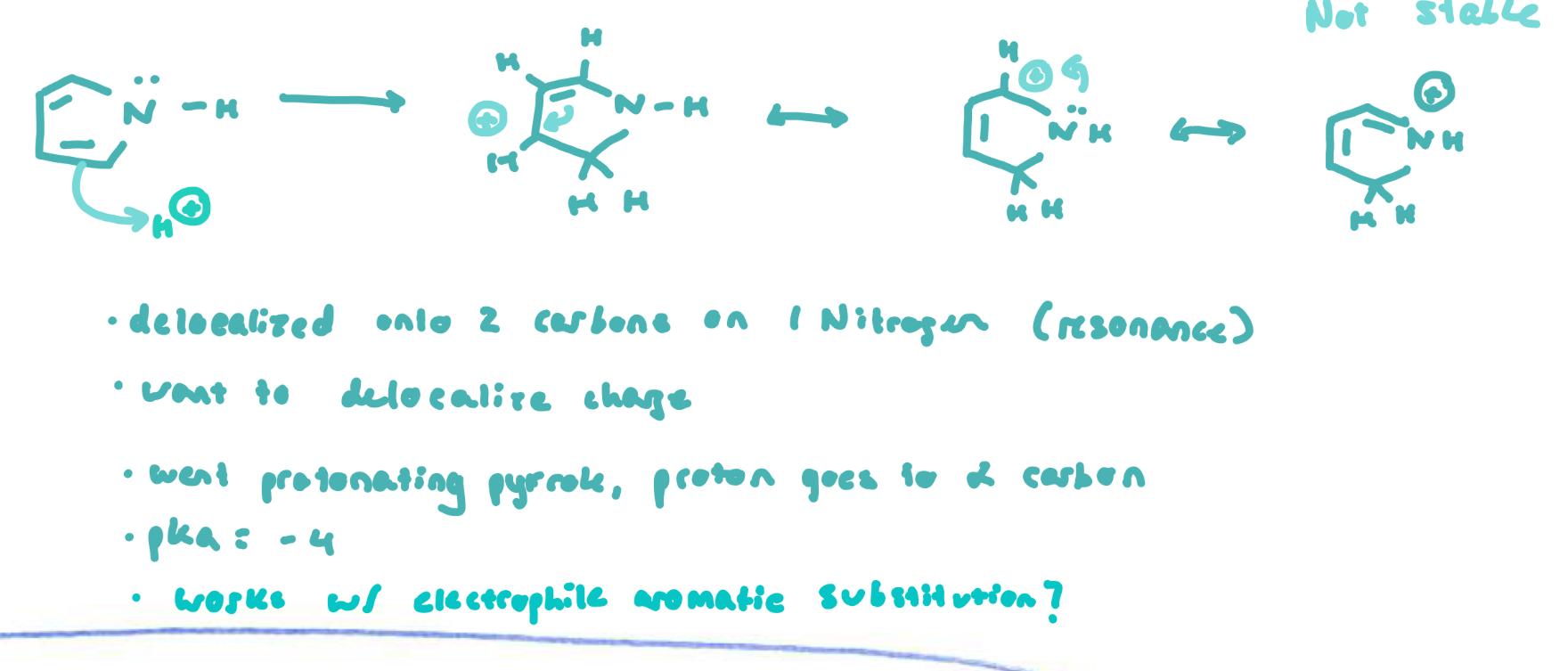
Pyrrolium
14 pKa units more acidic than ammonium due to a gain of aromaticity upon deprotonation to become pyrrole
if pyrrole is protonated on its N, it loses aromaticity and has no resonance structures
if it is protonated on its ⍺-carbon, it loses aromaticity and has 3 resonance structure
it does protonate on its ⍺-carbon over the N (and over the β-carbon, which has 2 resonance structures), but pyrrolium is very acidic
Guanidine
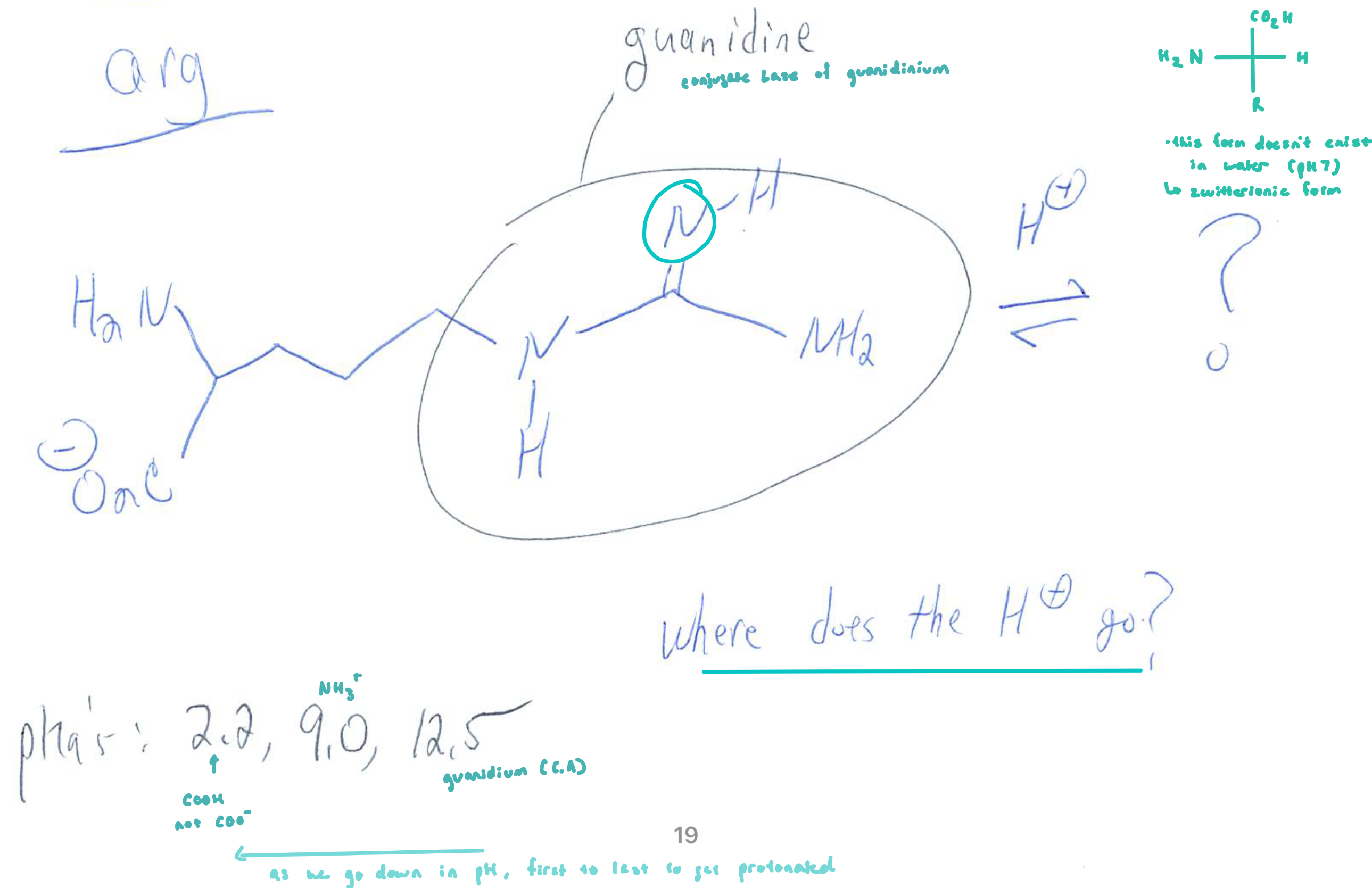
Guanidiniums (e.g. Arg)
have higher pKa’s than ammoniums due to delocalization of the (+) charge (resonance)
the conjugate acid is stabilized and thus less acidic
there is about 1/3 (+) charge on each N
there will only be a small charge on the carbon due to a lost octet on the carbon when it is charged
normally it is preferable to put a (+) charge on the less electroneg. atom
in guanidinium, it is better to put (+) charge on the more electroneg. N (and maintain all octets)
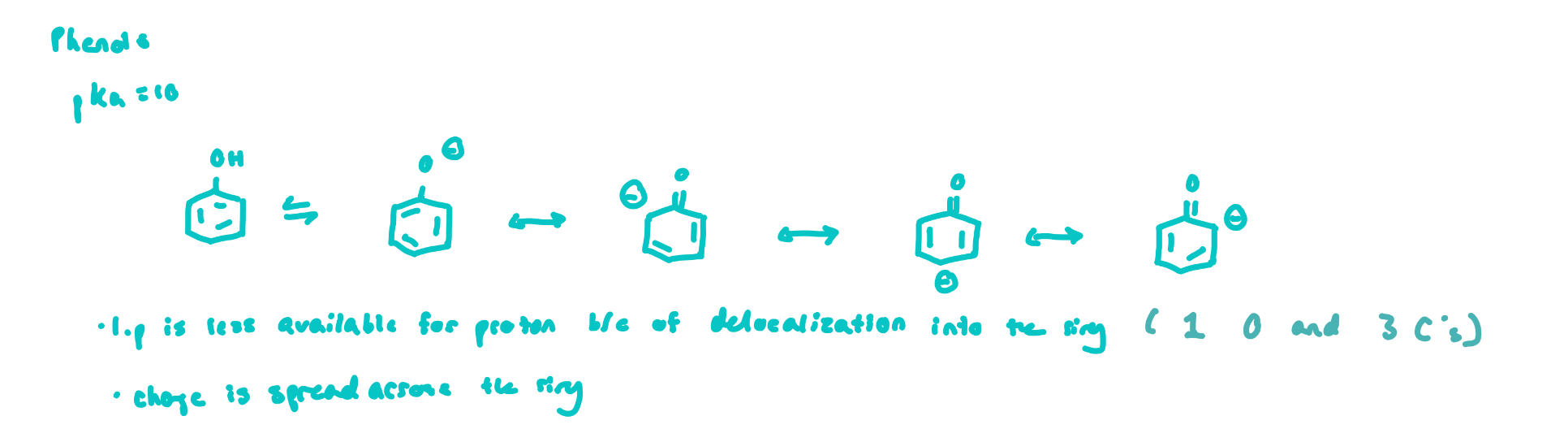
Phenols (e.g Tyr)
have lower pKa’s than alcohols due to delocalization of the (-) charge around the ring (resonance)
the conjugate base is stabilized
this breaks up aromaticity somewhat, but no octets are lost, so there is a net gain in stability of the conjugate base versus that of a simple alkoxide
Phenols Figure
Imidazole (His side chain)
aromatic
like pyridine, protonation does not disturb the aromaticity
also the l.p on the nitrogen(s) is sp2
an imidazolium should be a bit more stable than a pyridinium from the stand point of resonance b/w the nitrogens of the immidazolium, but the extra nitrogen also inducitvely withdraws → leads to overall similar pKa’s of imidazolium and pyridinium
Imidazole Figure

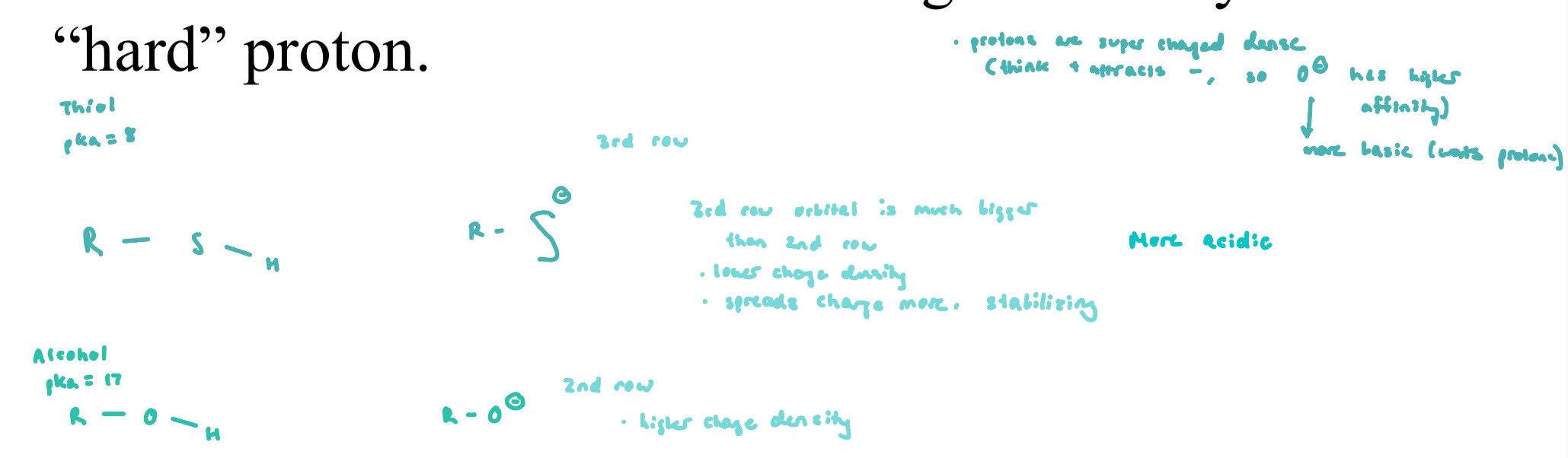
Thiols (Cys Side Chain)
pKa are much lower than alcohols due to lower charge density on anion
the “hard” alkoxide anion has higher affinity for a “hard” proton
Aromatic Compounds
An aromatic compound must:
be cyclic
have one unhybridized p-orbital on every atom in the ring (in the same orientation)
be planar
have 4n+2 pi electrons in the ring (where n=0, 1, 2, 3)
4n+2 can be 2,6,10, 14
Antiaromatic Compounds
Planar, cyclic, conjugated systems with 4n π electrons. They are unstable/reactive because the MO diagram has two unparied electrons
Nonaromatic Compounds
Nonaromatic compounds fail any one of Hückel’s rules.
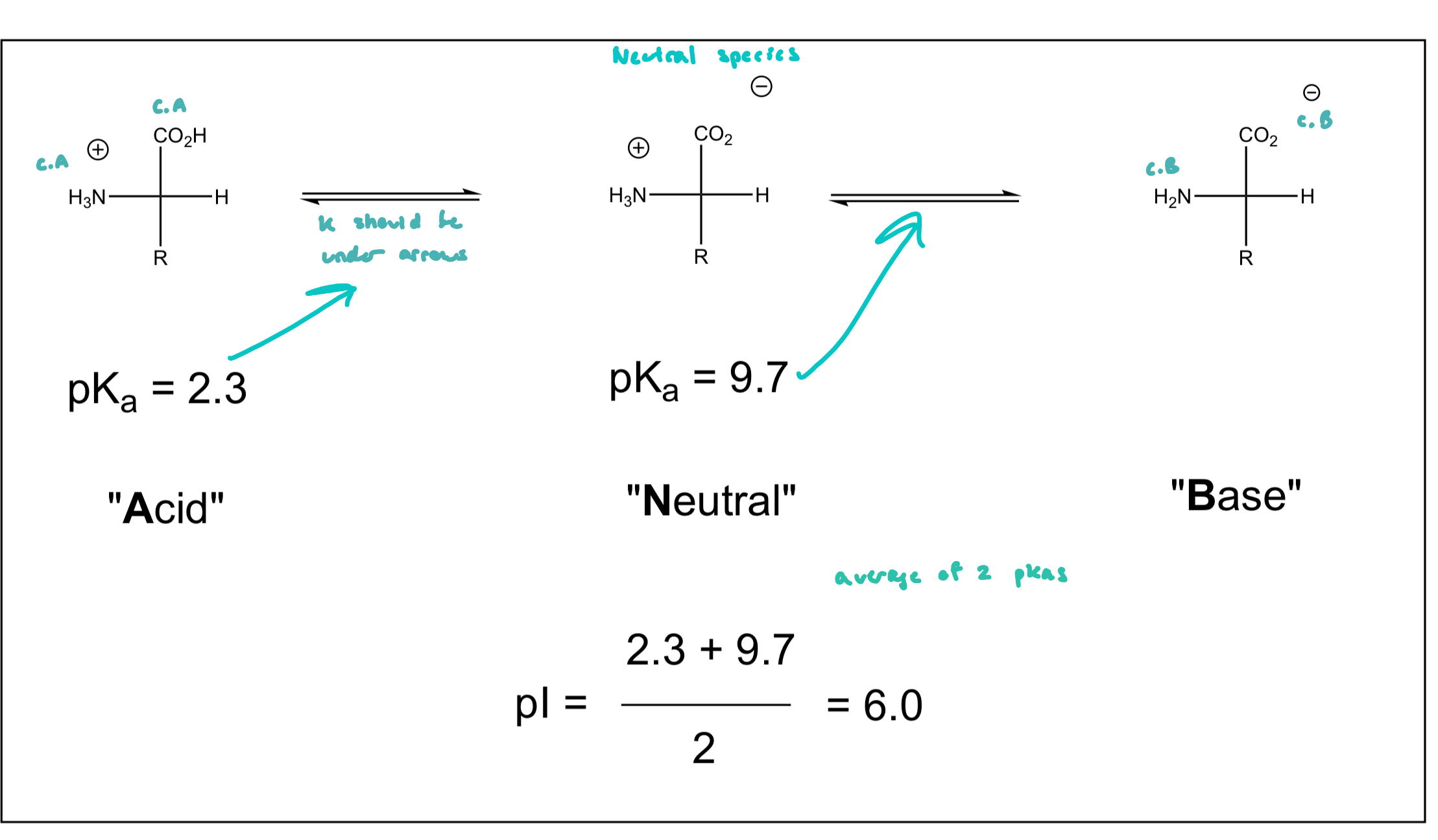
Isoelectric Point (pI)
the pH at which the a.a. exists in solution predominantly as a neutral species
for simple a.a.’s (those w/ no acidic or basic groups in their side chains)
pI = (pKa1 + pKa2)/2
average of two pKa’s
if there is a potential charge in the side chain, the pI is the average of the two pKas that yield a neutral species
Buffers
if a.a’s are present in appreciable amounts, they can be used as buffers
they can buffer at any pH that corresponds to one of their pKa’s