Chapter 4: Carbon and the Molecular Diversity of Life
Carbon: The Backbone of Life
- living organisms consists mostly of carbon-based compounds
- carbon in unparalleled in its ability to form large, complex, and varied molecules
- makes possible the diversity of organisms that have evolved on Earth
- Proteins, DNA, carbohydrates, and other molecules that distinguish living matter are all composed of carbon compounds
Concept 4.1: Organic chemistry is the study of carbon compounds
- organic chemistry: the study of compounds that contain carbon, regardless of origin
- range from simple molecules to colossal ones, which as proteins
^^Organic Molecules and the Origin of Life on Earth^^
- Stanley Miller’s classic experiment, trying to model how life first began, demonstrated the abiotic synthesis of organic compounds
- experiments support the idea that abiotic synthesis of organic compounds, perhaps near volcanoes, could have been a stage in the origin of life
- the overall percentages of the major elements of life — C, H, O, N, S, P — are suite uniform from one organism to another
- because of carbon’s ability to form four bonds, these building blocks can be used to make an inexhaustible variety of organic molecules
- the great diversity of organisms on the planet is due to the versatility of carbon
Concept 4.2: Carbon atoms can form diverse molecules by bonding to four other atoms
- electron configuration is the key to an atom’s characteristics
- electron configuration determines the kinds and number of bonds an atom will form with other atoms
- we are carbon based, not silicon based because silicon holds onto other elements too strongly - can’t let go of elements easily when elements need to be used by the organism
^^The Formation of Bonds with Carbon^^
- carbon has 4 valence electrons, so it can form four covalent bonds with a variety of atoms, making large complex molecules possible
- in molecules with multiple carbons, each carbon bonded to four other atoms has a tetrahedral shape
- when two carbon atoms are joined by a double bond, the atoms joined to the carbons are in the same plane as the carbons
- double bonds don’t allow carbons to freely rotate (not dynamic like single bonds)
- ==valence==: the number of covalent bonds an atom can form
- number of unpaired electrons in the valence shell of an atom is generally equal to its valence
- the electron configuration of carbon gives it covalent compatibility with many different elements
- the valence of carbons and its most frequent partners (hydrogen, oxygen, and nitrogen) are the building code for the architecture of living molecules
^^Molecular Diversity Arising from Variation in Carbon Skeletons^^
- carbon chains form the skeletons of most organic molecules and they vary in length and shape
- variation in carbon skeletons is an important source of the molecular complexity and diversity of living matter
@@Hydrocarbons@@
- ==hydrocarbons==: organic molecules consisting of only carbon and hydrogen
- many organic molecules, such as fats, have hydrocarbon components
- but, hydrocarbons are not prevalent in most living organisms
- hydrocarbons can undergo reactions that release a large amount of energy
- fat is more efficient to break down than sugar
@@Isomers@@
- ==isomers==: compounds with the same molecular formula but different structures and properties
- ==structural isomers==: have different covalent arrangements of their atoms
- ==cis-trans isomers==: have the same covalent bonds, but differ in their spatial arrangements
- ==enantiomers== : isomers that are mirror images of each other
- cis-trans isomers require a double or triple bond and react differently in reactions
- enantiomers are important in the pharmaceutical industry as two enantiomers of a drug may have different effects
- usually only one isomer is biologically active
- single bonded molecules will react the same, even if their bonds are rotated
- double-bonded or triple-bonded molecules will react different in reactions if their bonds are rotated
Concept 4.3: A few chemical groups are key to molecular function
- the properties of an organic molecule depend not only on the arrangement of its carbon skeleton but also on the various chemical groups attached to the skeleton
- a number of characteristic groups can replace hydrogens attached to skeletons of organic molecules
^^The Chemical Groups Most Important in the Processes of Life^^
estradiol and testosterone are both steroids with a common carbon skeleton, in the form of four used rings
these sex hormones differ only in the chemical groups attached to the rings of the carbon skeleton
you would think estrogen and testosterone are a antagonist chemicals, but they’re actually pretty similar - only differ by groupings that they have
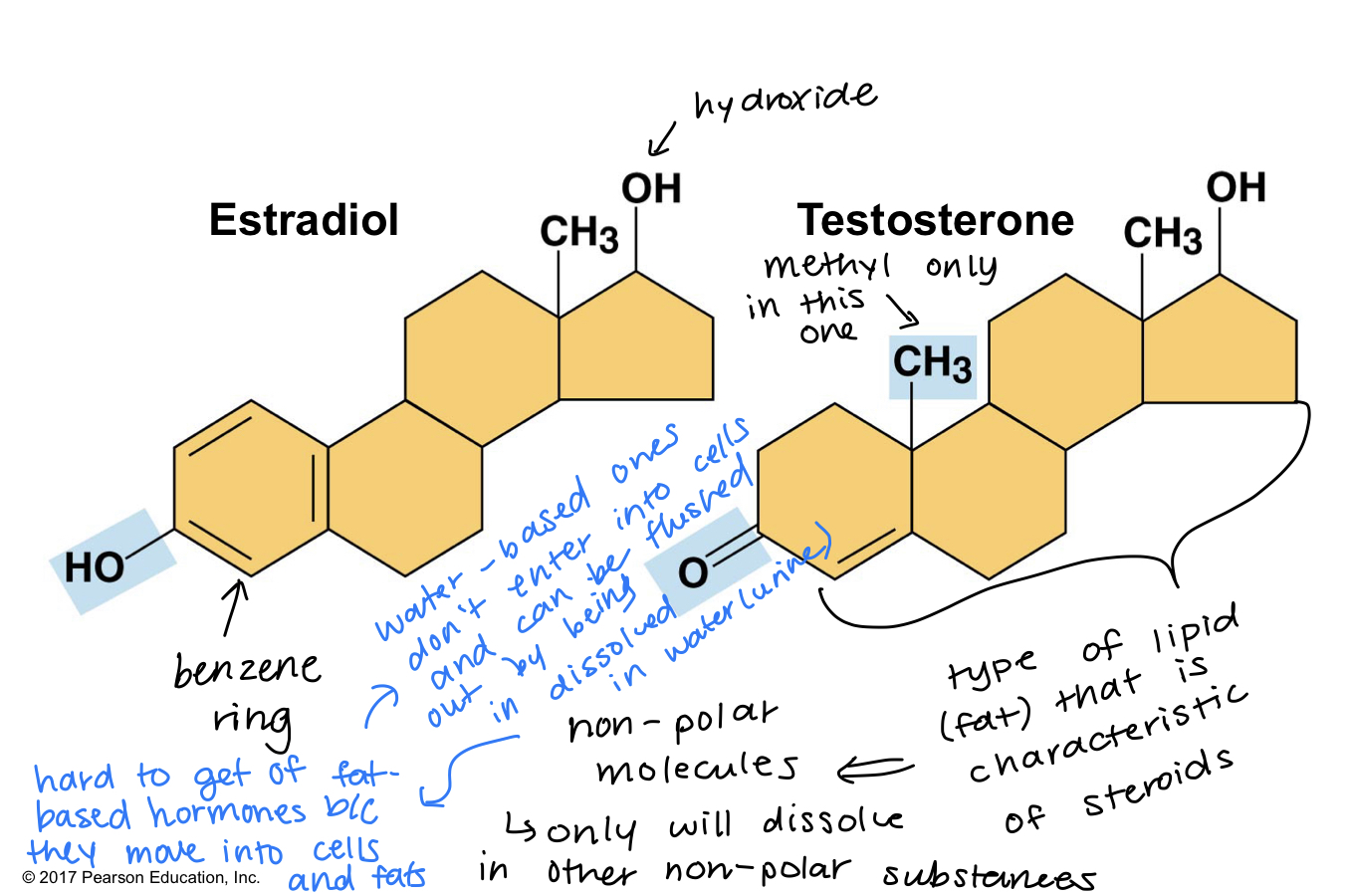
==functional groups==: the components of organic molecules that are most commonly involved in chemical reactions
the number and arrangement of functional groups gives each molecule its unique properties
^^ATP: An Important Source of Energy for Cellular Processes^^
- ==Adenosine Triphosphate (ATP)==: an important organic phosphate that is the basis of life
- ATP consists of an organic molecule called adenosine attached to a string of three phosphate groups
- ATP is made in the inner-lining of the mitochondria
- ATP is not a very stable molecule, allowing it to form ADP and release a phosphate group that then become inorganic