Addition rxn to Alkenes- orgo 1
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Hydrohalogenation
Halogen goes to more substituted carbon of alkene. BE CAREFUL OF CARBOCATION REARRANGEMENT

alkene reacts with HX (X= F, Cl, Br, I)
hydrohalogenation rxn where halogen goes to more substituted carbon, forming a carbocation. (watch out for rearrangement)
Halogenation
a halogen goes on each carbon of the alkene
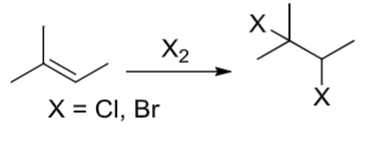
An alkene reacts with X2 (X=Cl, Br)
Halogenation rxn where the 2 halogens go on each carbon of the alkene
Hydration
Weak base (alcohol or water) adds to most substituted carbon of the alkene BE CAREFUL OF CARBOCATION REARRANGEMENT
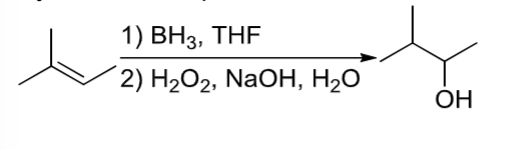
An alkene reacts with H+ and ROH (R= alkyl, aryl, alkenyl, H)
Hydration rxn where alcohol or water is added to most substituted carbon of alkene BE CAREFUL OF CARBOCATION REARRANGEMENT
Hydroboration/Oxidation
OH is added to less substituted carbon of alkene through a two-step process involving BH3,THF and H2O2, NaOH, OH-.
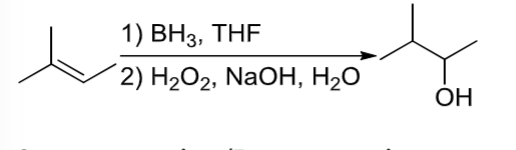
An alkene reacts in a 2 step rxn with step 1) BH3, THF and step 2) H2O2, NaOH, OH-
Hydroboration/Oxidation rxn where alcohol is added to the less substituted carbon of the alkene.
Oxymercuration/Demercuration
OH is added to the more substituted carbon of the alkene (like hydration, but with no possibility of rearrangement)
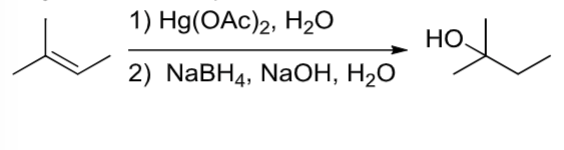
an alkene reacts in a 2 step rxn with step 1)Hg(OAc)2, H2O and step 2) NaBH4, NaOH, H2O
Oxymercuration/Demercuration rxn where OH is added to more substituted carbon of alkene
Radical Hydrohalogenation
Halogen adds to less substituted carbon of alkene through 3 step radical mechanism
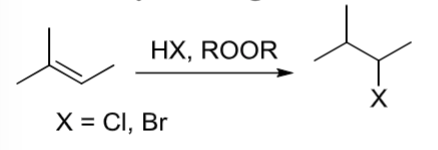
an alkene reacts with HX, ROOR (X=Cl, Br)
Radical Hydrohalogenation rxn with 3 step mechanism where Halogen adds to less ubstituted carbon of alkene
Radical Polymerization
Alkene becomes repeating unit of polymer. (Only two carbons will be in the parentheses and whatever groups are bound to the alkene).
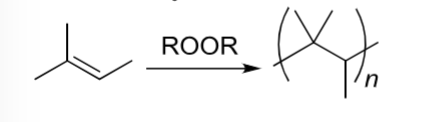
an alkene reacts with only ROOR
Radical Polymerization rxn where alkene becomes repeating unit of polymer
Radical Halogenation of Alkanes
Halogen goes on most substituted carbon of alkane through 3 step radical mechanism
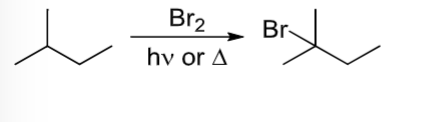
An alkane reacts with Br2 and heat or light
Radical Halogenation of alkanes rxn halogen goes on must substituted carbon via a three-step radical mechanism.