Changing atomic model: Atomic structure and the periodic table: Chemistry: GCSE (9:1)
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Ancient greek model of the atom
Atoms are tiny solid spheres which cannot be divided.
JJ Thompson
Discovered the electron and developed the "plum-pudding" model of the atom
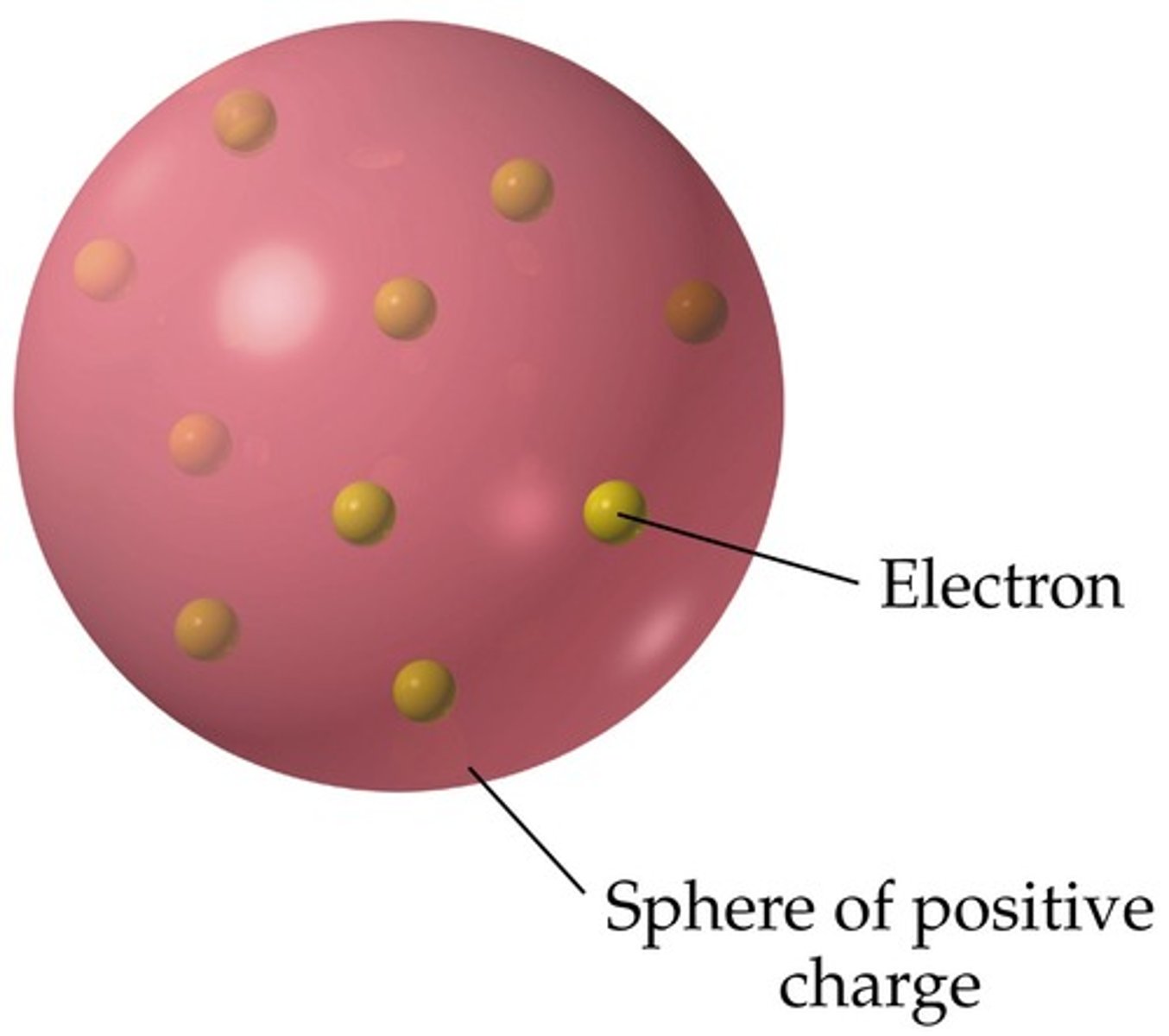
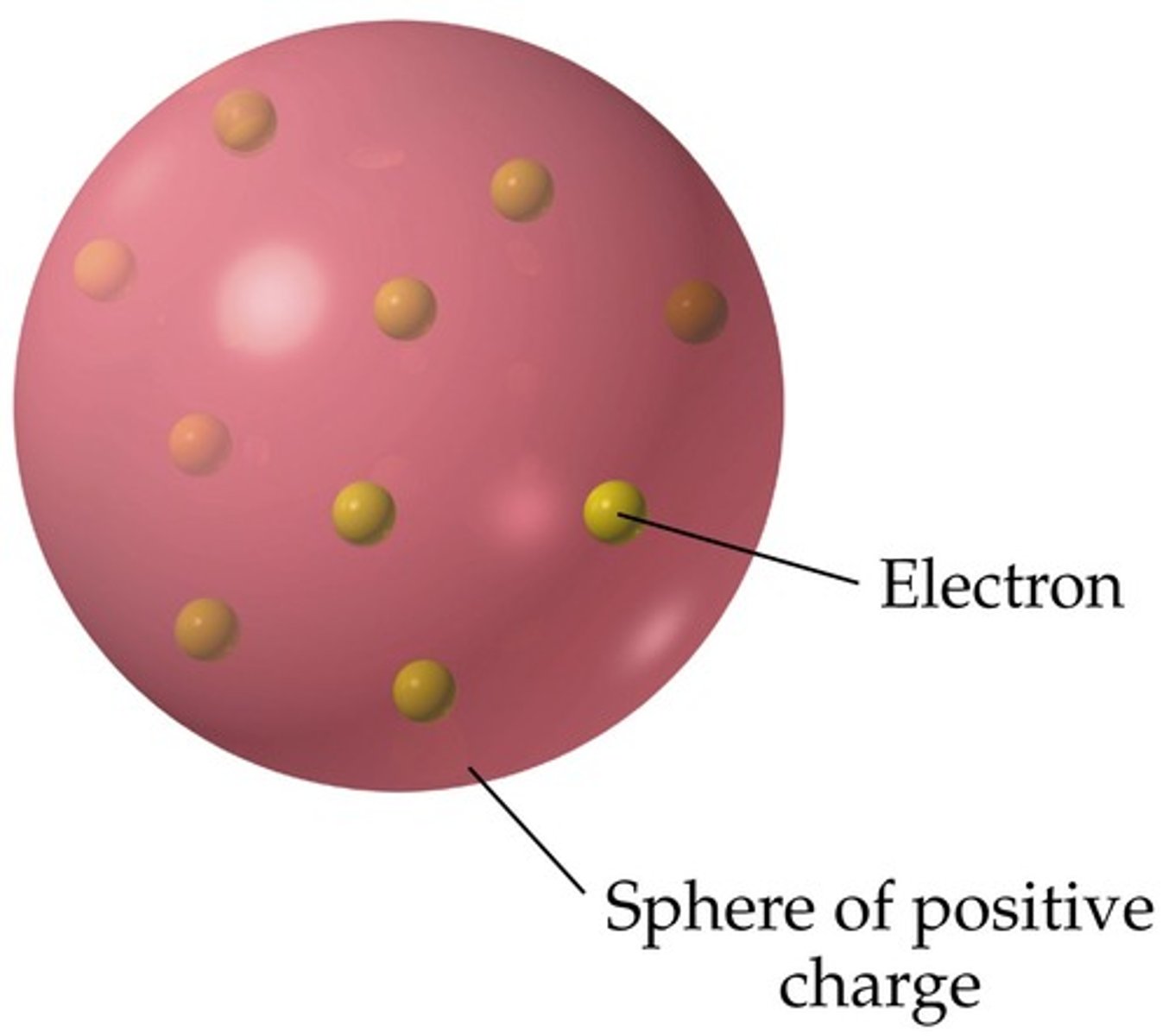
plum pudding model of the atom
atoms are balls of positively charge with negative electrons embedded in it
Rutherford, Geiger, and Marsden
carried out the alpha particle scattering experiment and developed the nuclear model of the atom
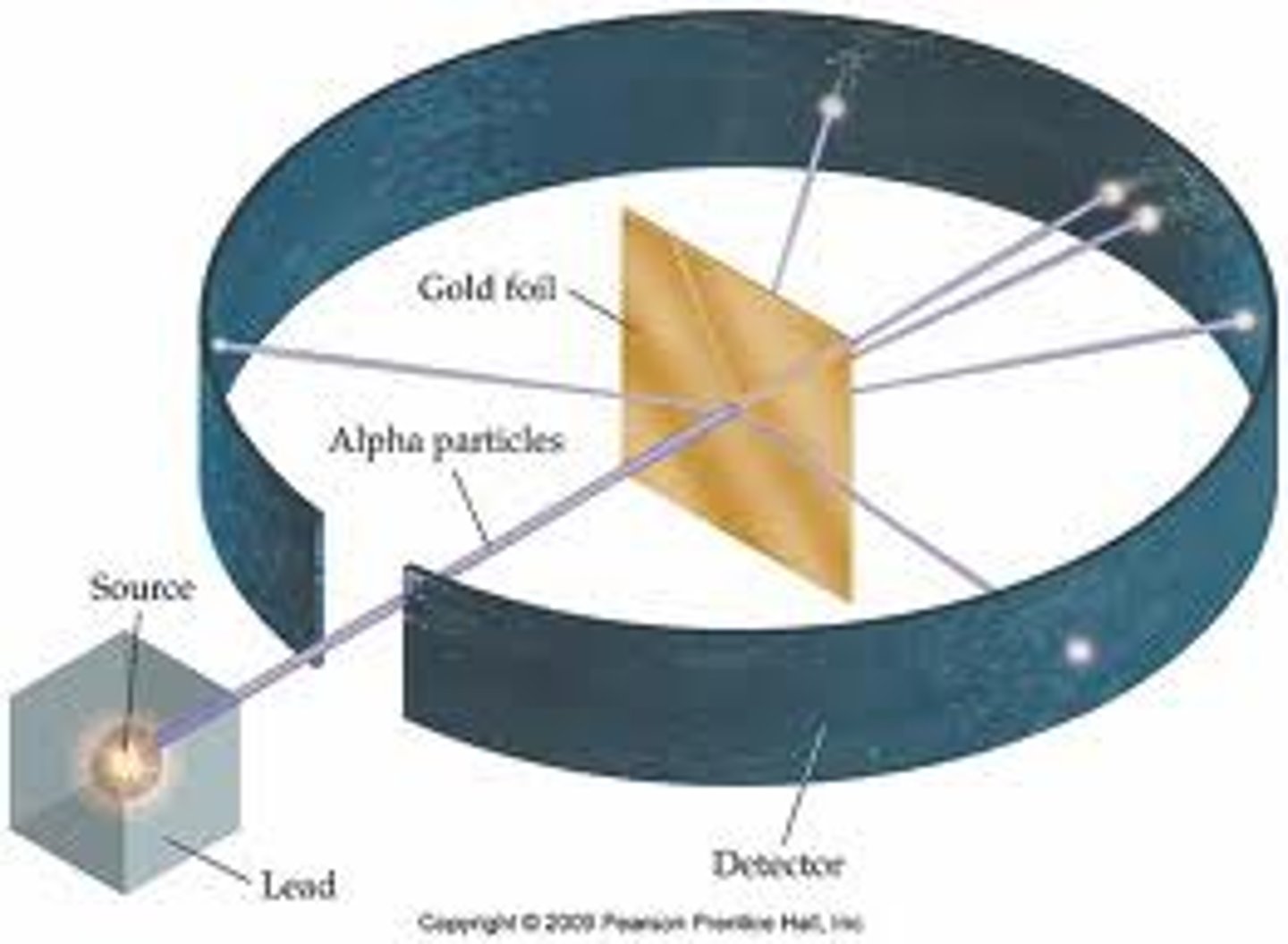
Alpha particle scattering experiment
Fired alpha particles at a thin sheet of gold foil.
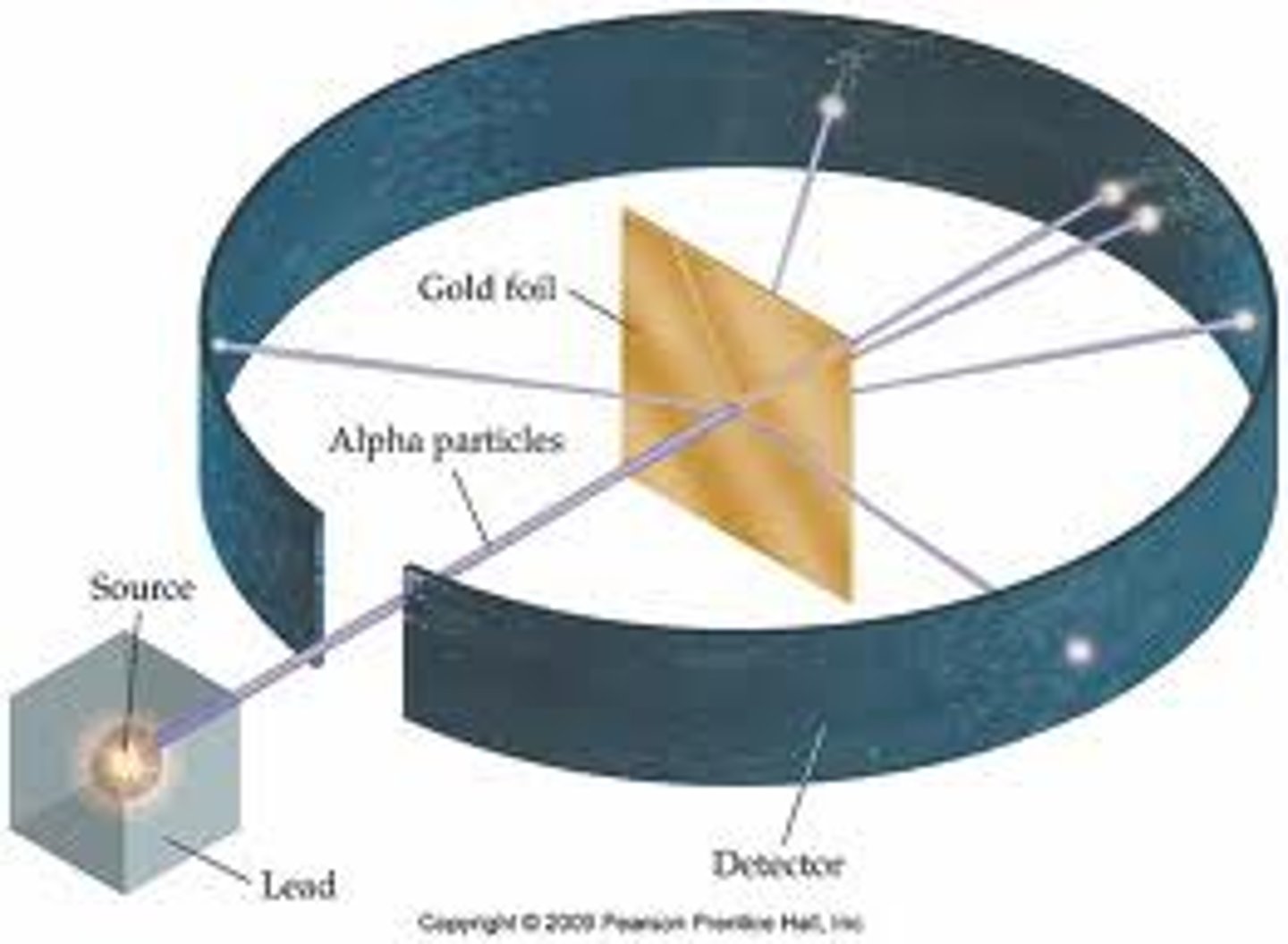
Conclusions of the alpha particle scattering experiment
Most of the atom is empty space except for a tiny positive nucleus.
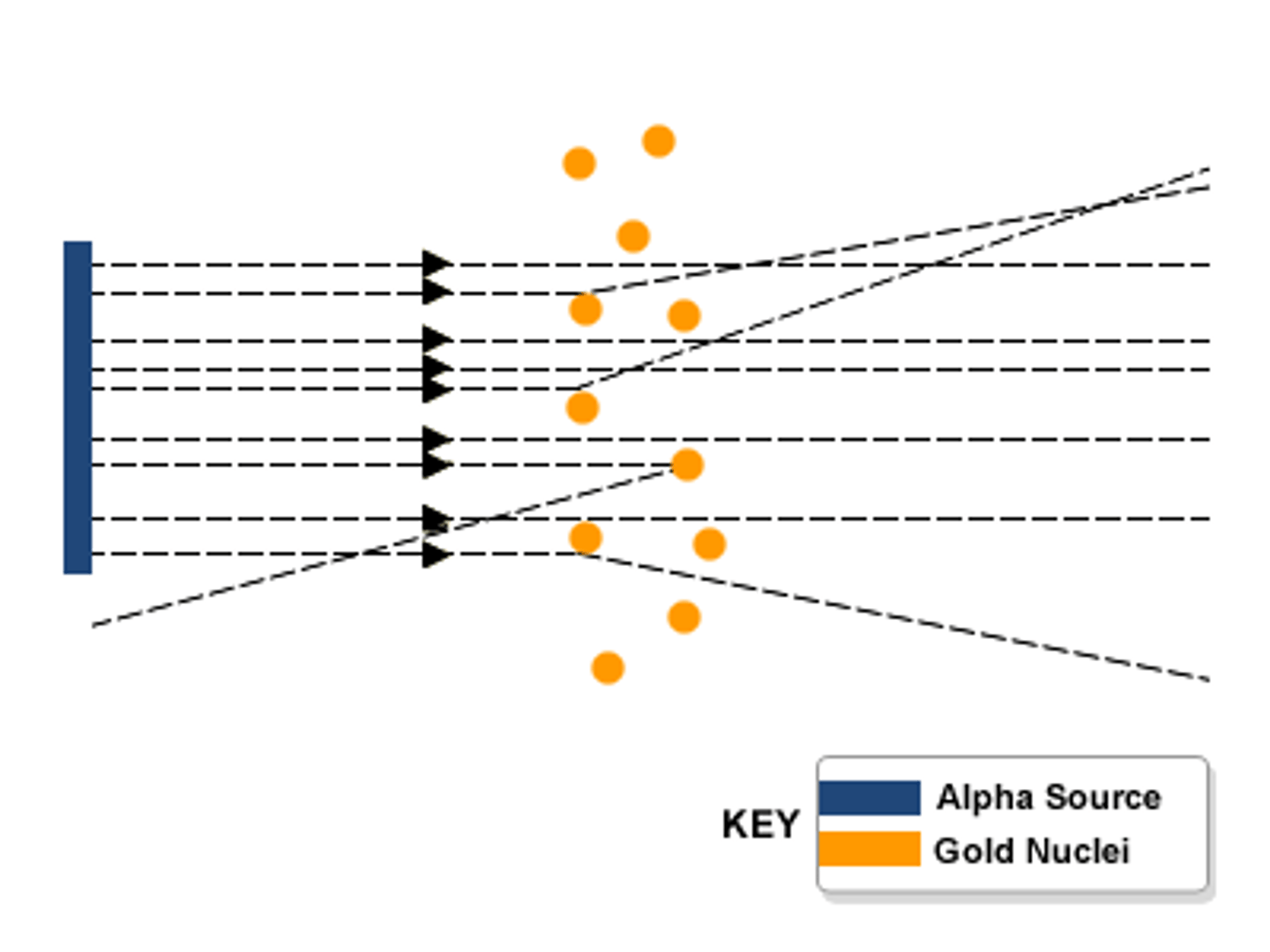
Observations of the alpha particle scattering experiment
Most of the alpha particles went straight through the atom, a few alpha particles bounced back.
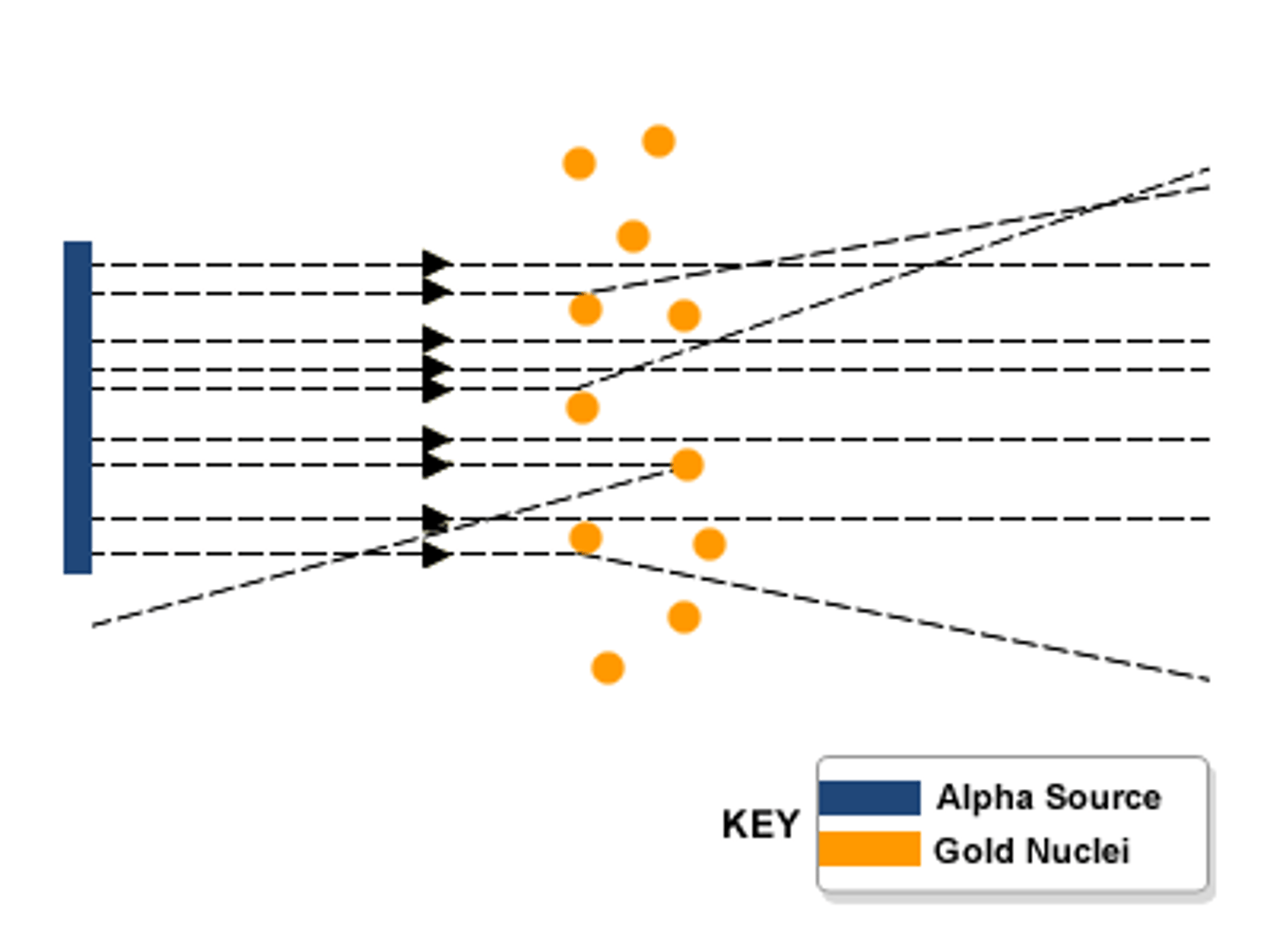
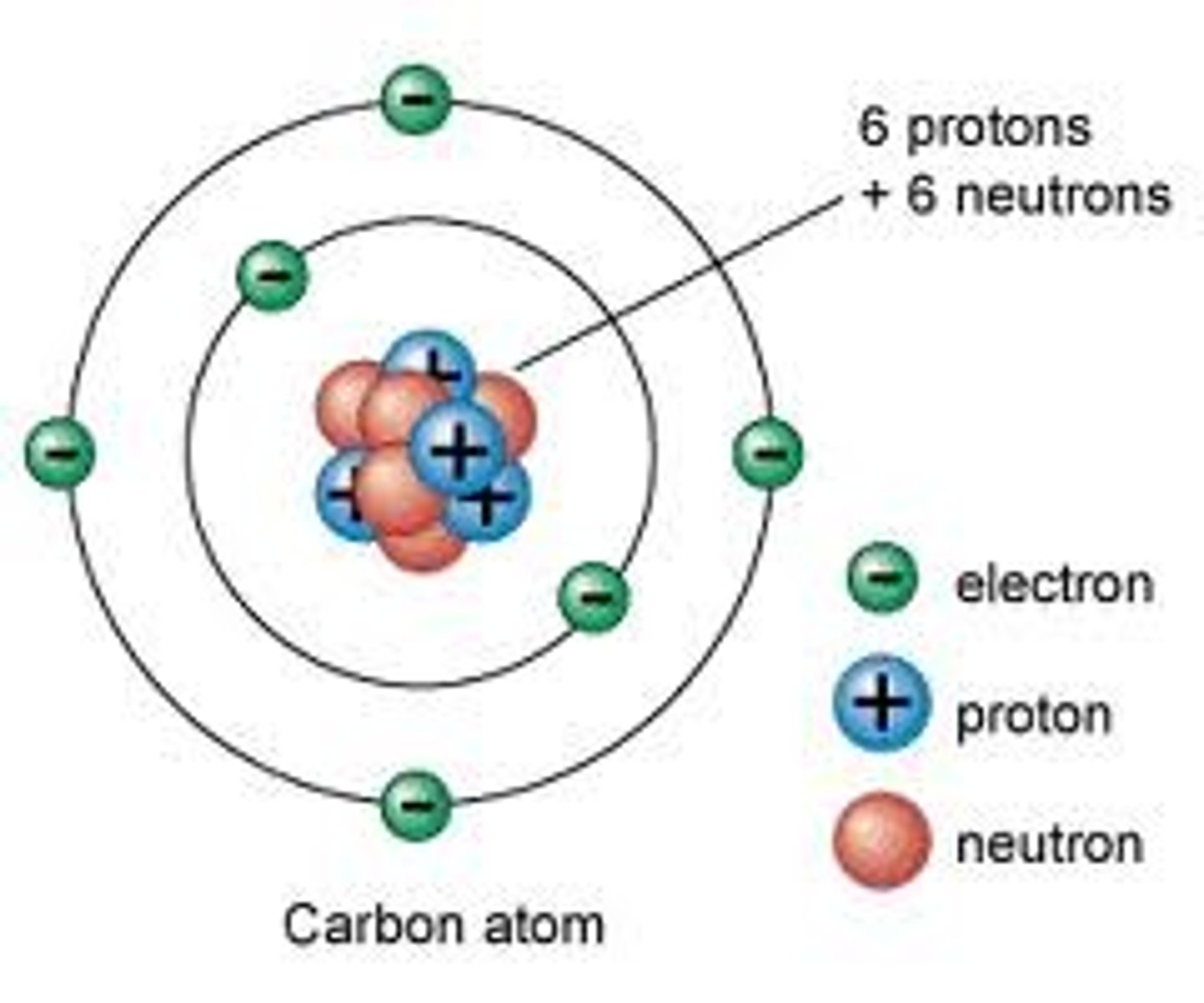
Nuclear model of the Atom
Atoms are made of a small positive nucleus orbited by negative electrons.
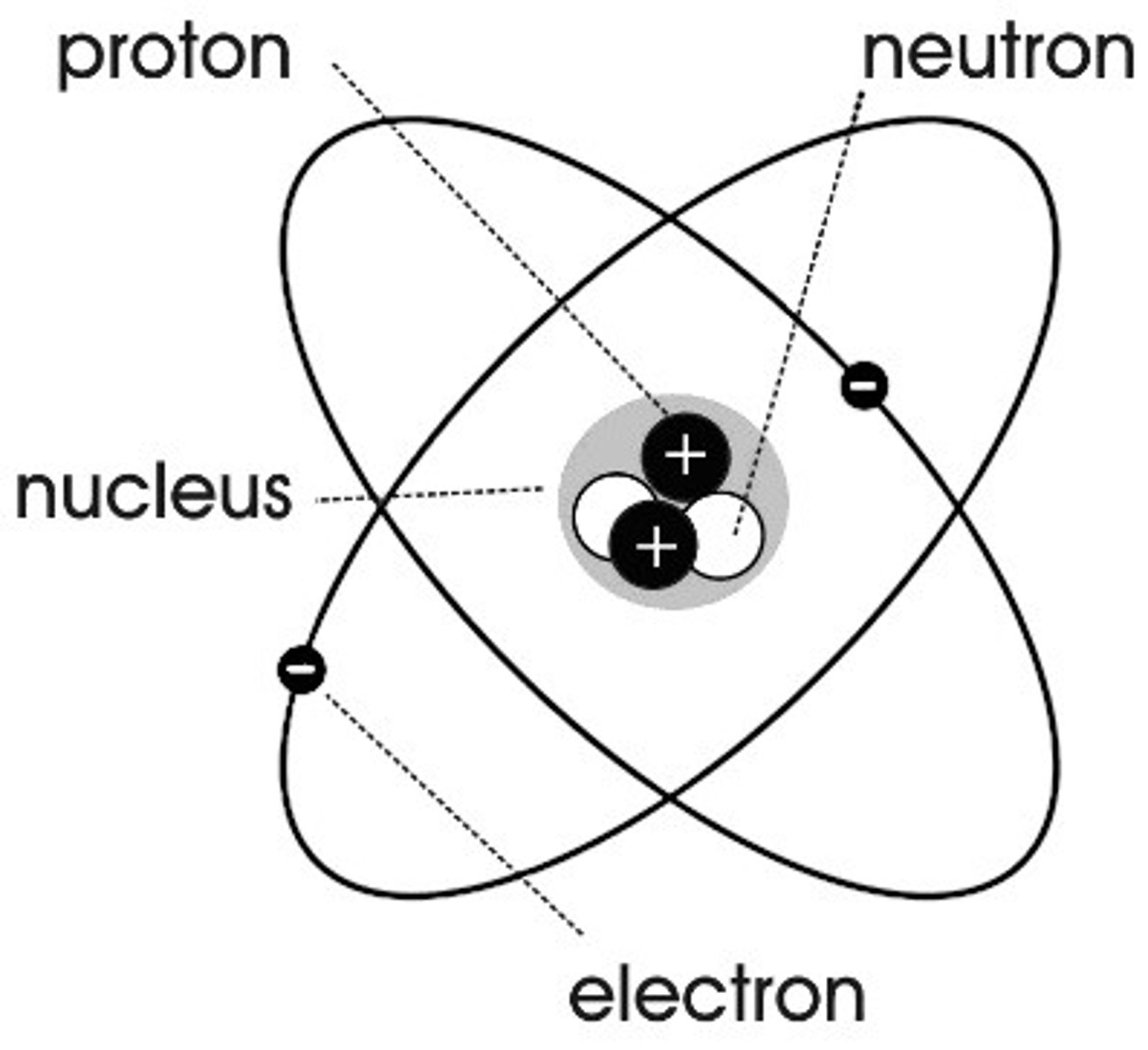
Niels Bohr
Adapted the nuclear model, by suggesting that electrons orbit the nucleus at specific distances.
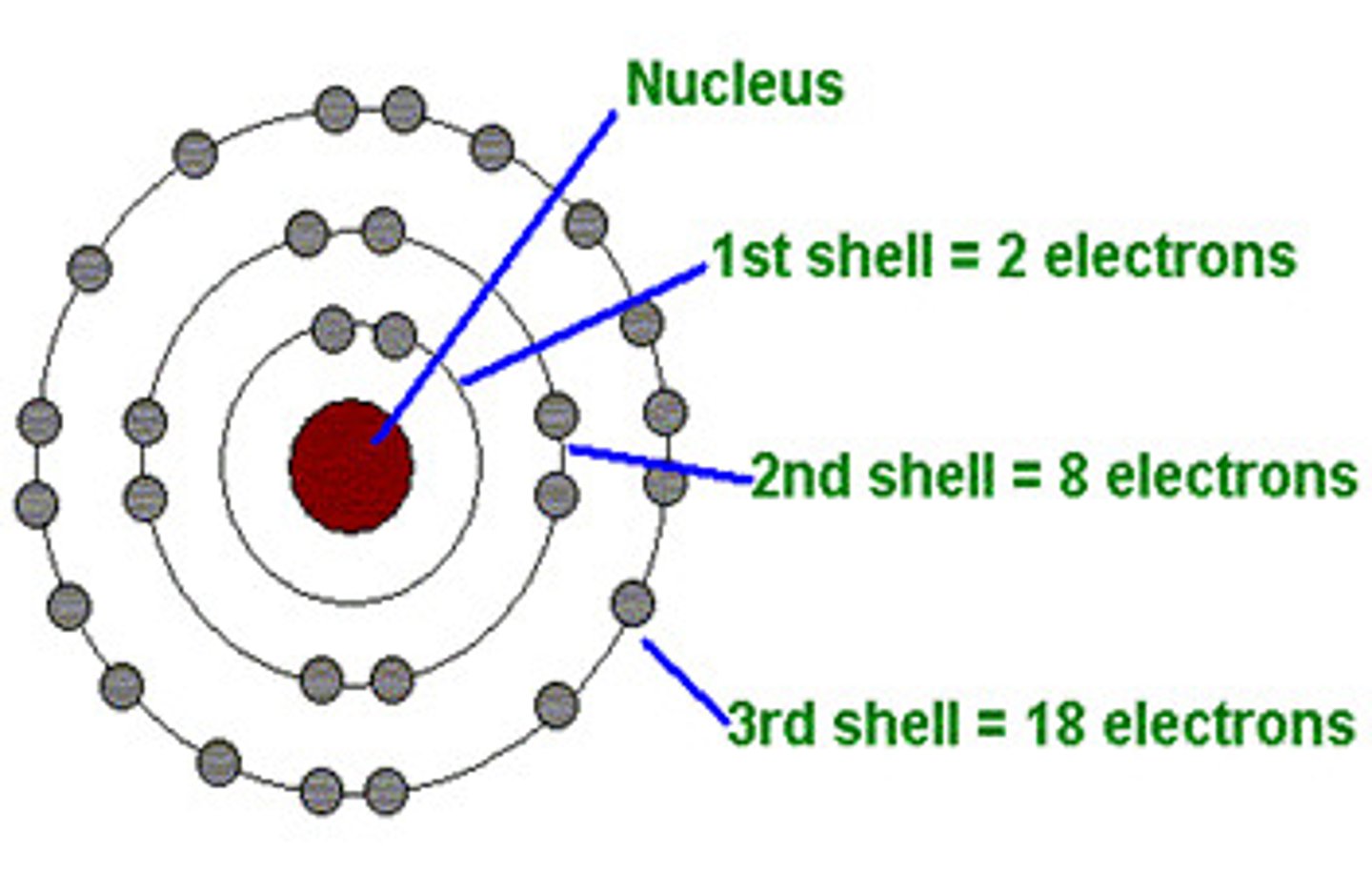
James Chadwick
Carried out experiments which provided evidence of neutrons.
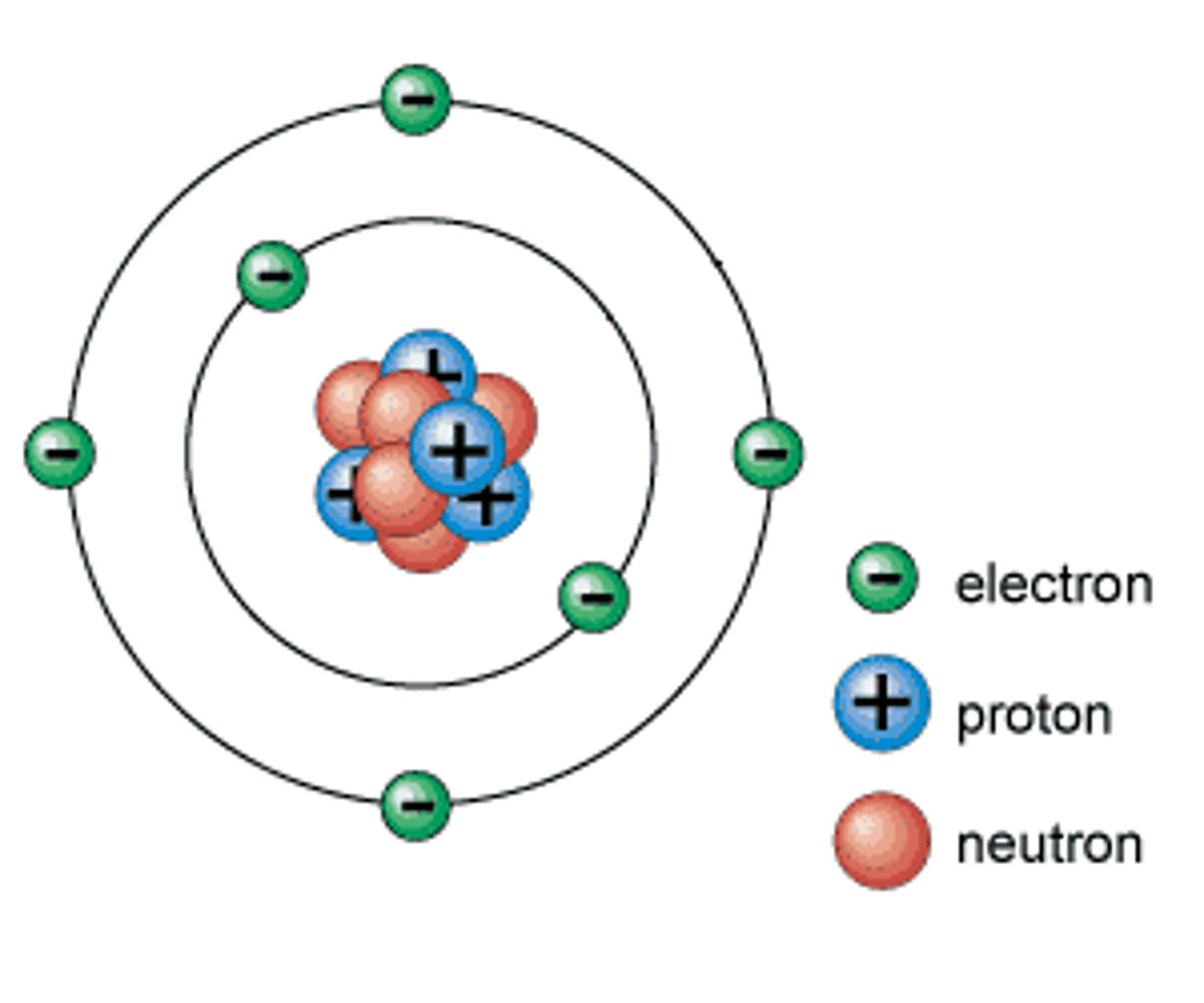
Proton
A subatomic particle that has a positive charge and that is found in the nucleus of an atom.
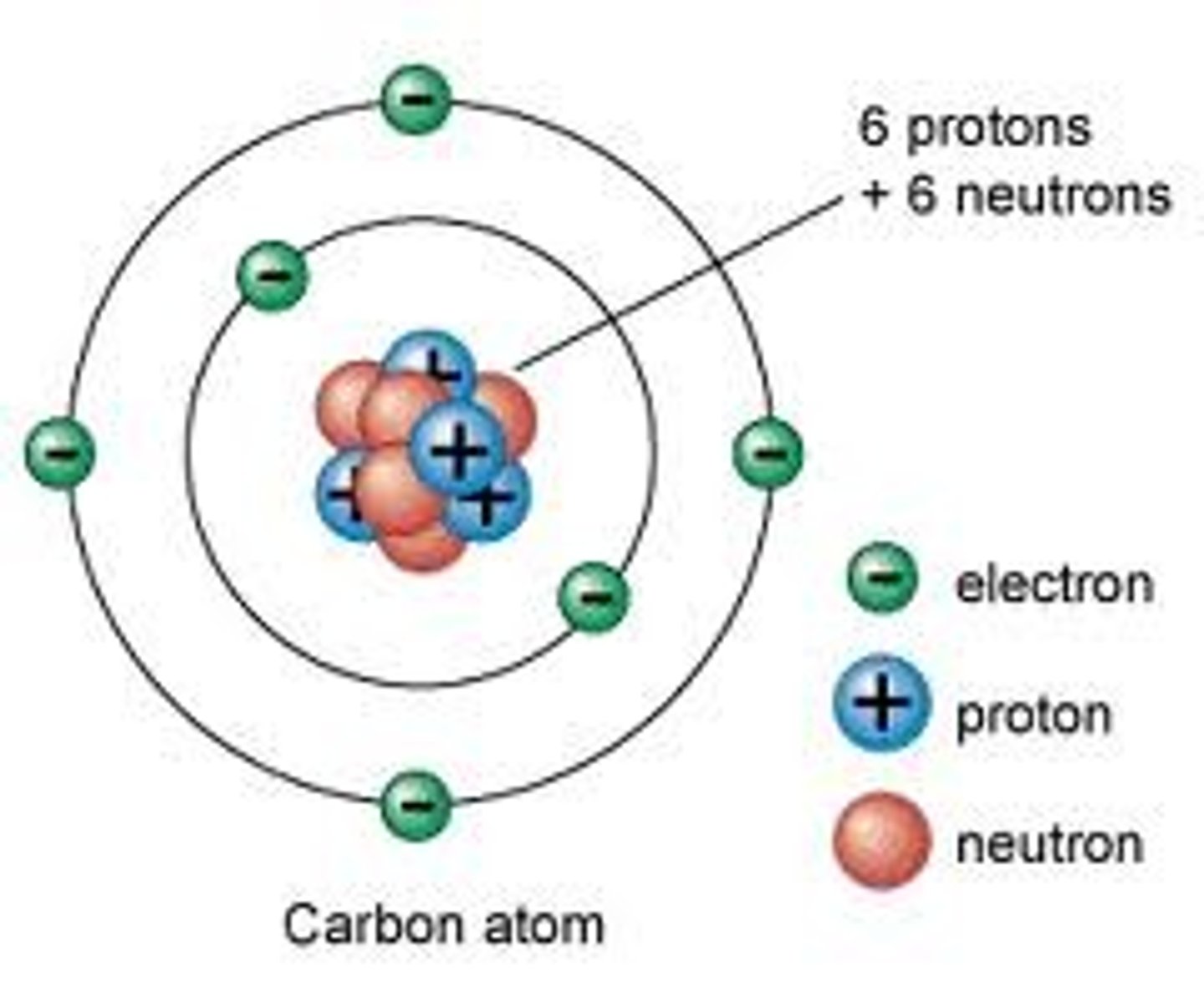
Neutron
A subatomic particle that has no charge and is found in the nucleus of an atom.
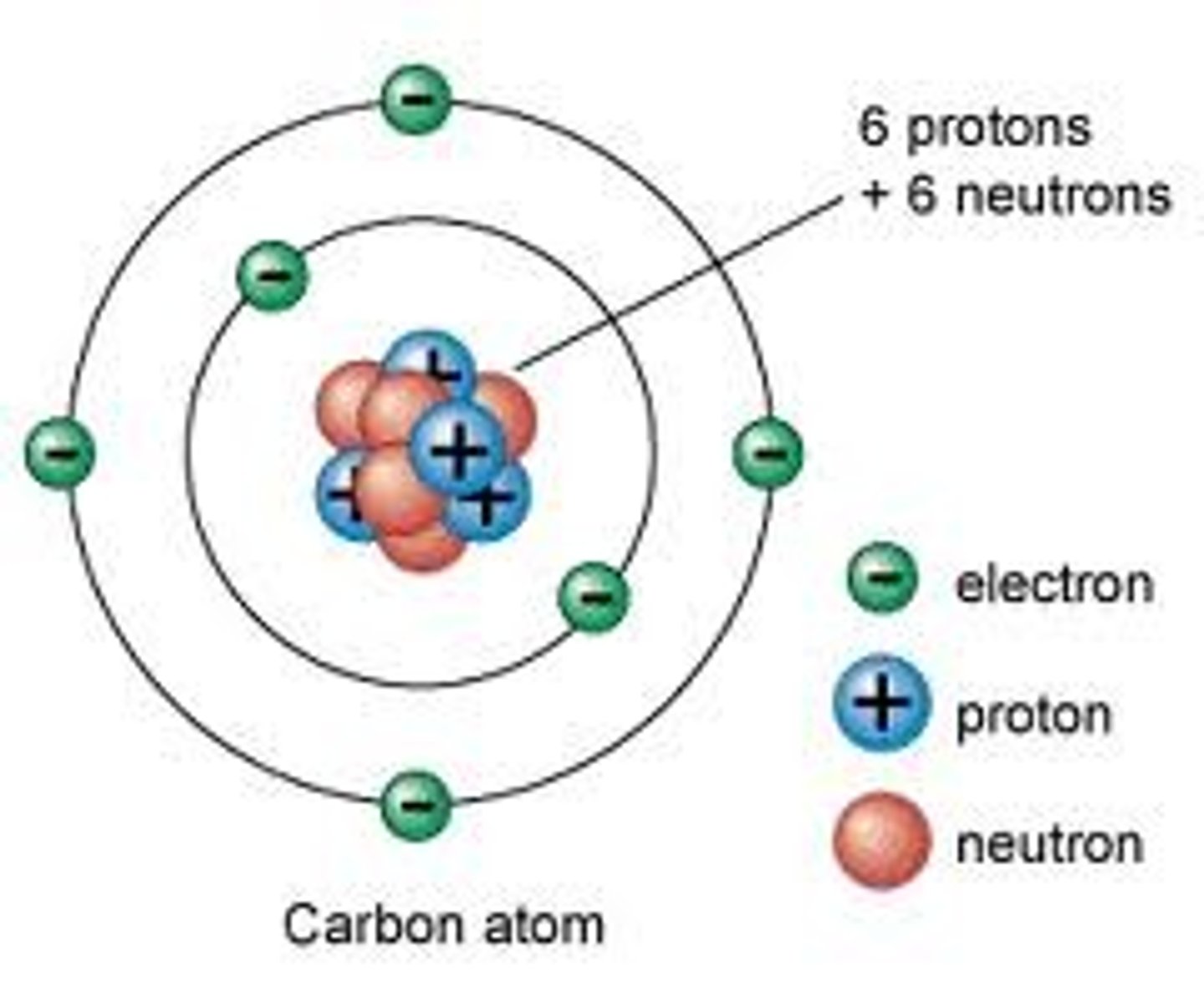
Electron
A subatomic particle that has a negative charge and orbits the nucleus in shells.