Lesson 10: Applications of Electrolytic Cells
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Last updated 7:47 PM on 10/30/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
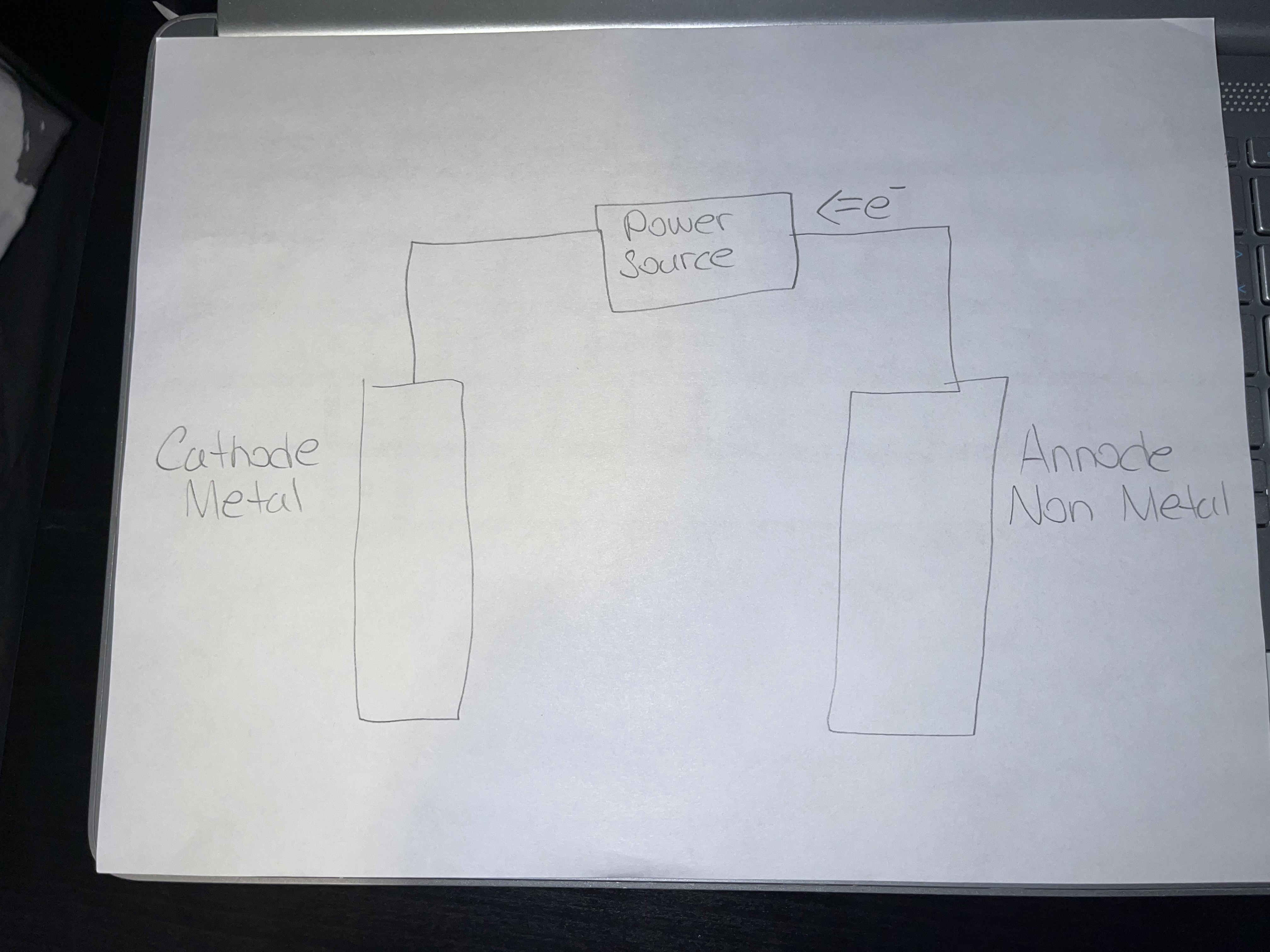
Production of Elements/Molten Salt Electrolysis
Requires molten ionic compounds
Forms metals at the cathode
Forms non metals at the anode
2
New cards
Molten
Some metals react with water which is why it isn’t aqueous
3
New cards
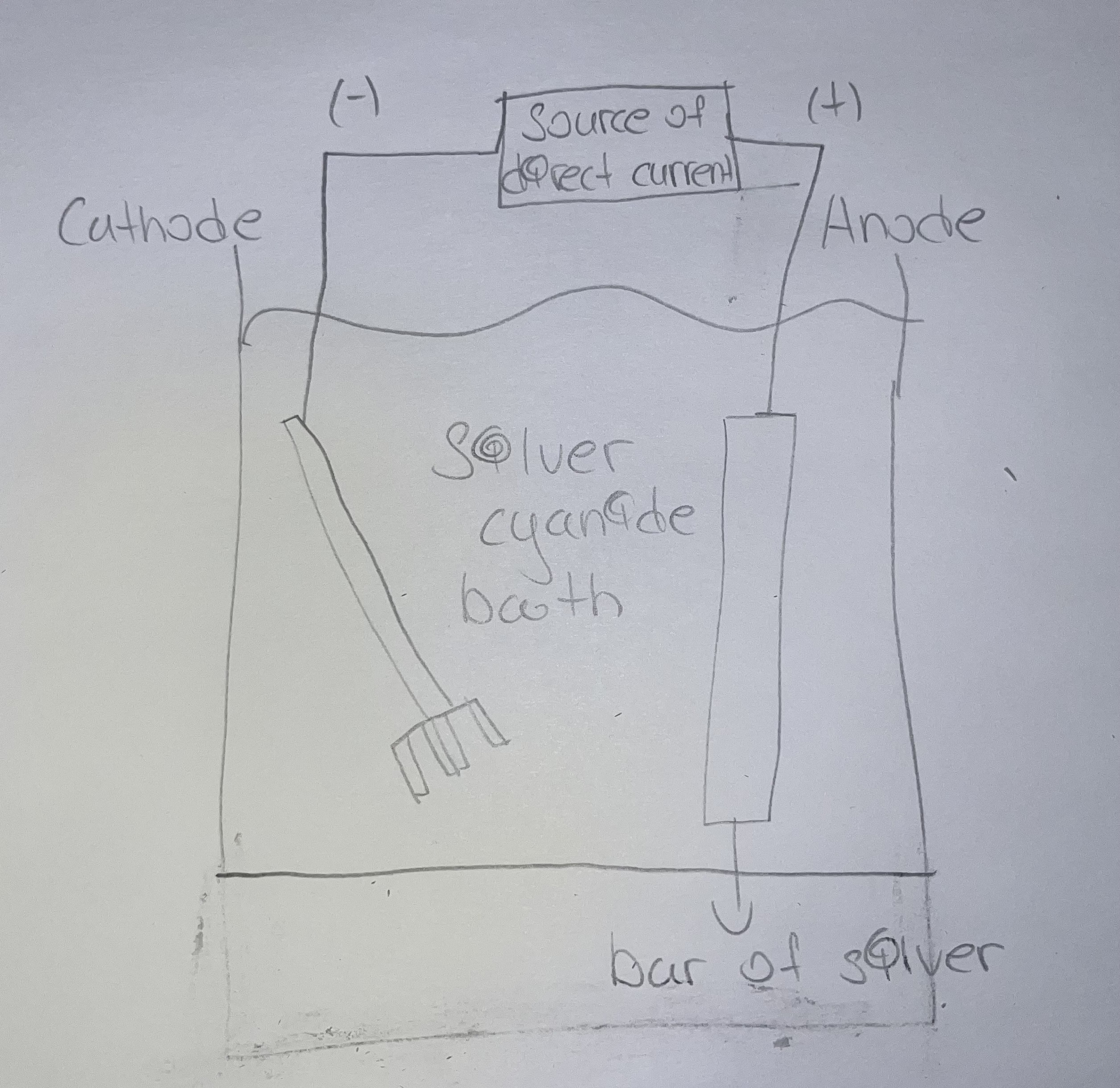
Refining of Metals (Electrofining)
Impure solid metals are oxidized to aqueous cations
Anode = impure metal
Cathode = pure metal
4
New cards
Electroplating
The process of covering one metal with another
Cover the cathode (CC) to protect from rusting
Chroming is commonly used on cars