Lesson 9: Electrolytic Cells, Chloride Anomaly, Change of Reference Half Cell
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
9 Terms
Standard Reference Half Cell
E not cell half reaction can’t be determined
Voltmeters measure the diff between two half cells
Zero point is chosen to comapre other half reactions
Electrolytic Cell
External power source used to make a non spontaneous redox reaction
Involves two electrodes and an electrolyte
Electrolysis
Forcing a non spontaneous redox reaction by supplying electrical NRG
External power source acts like an electron pump using electrical NRG to do work on electrons and promote electron transfer
Procedure to Analyze Electrolytic Cells
Identify SOA and SRA
Write half reactions
Balance electrons and write net cell reaction + cell potential
IF required state the minimum electric potential (V)
If diagram is needed write one down (shown on pic)
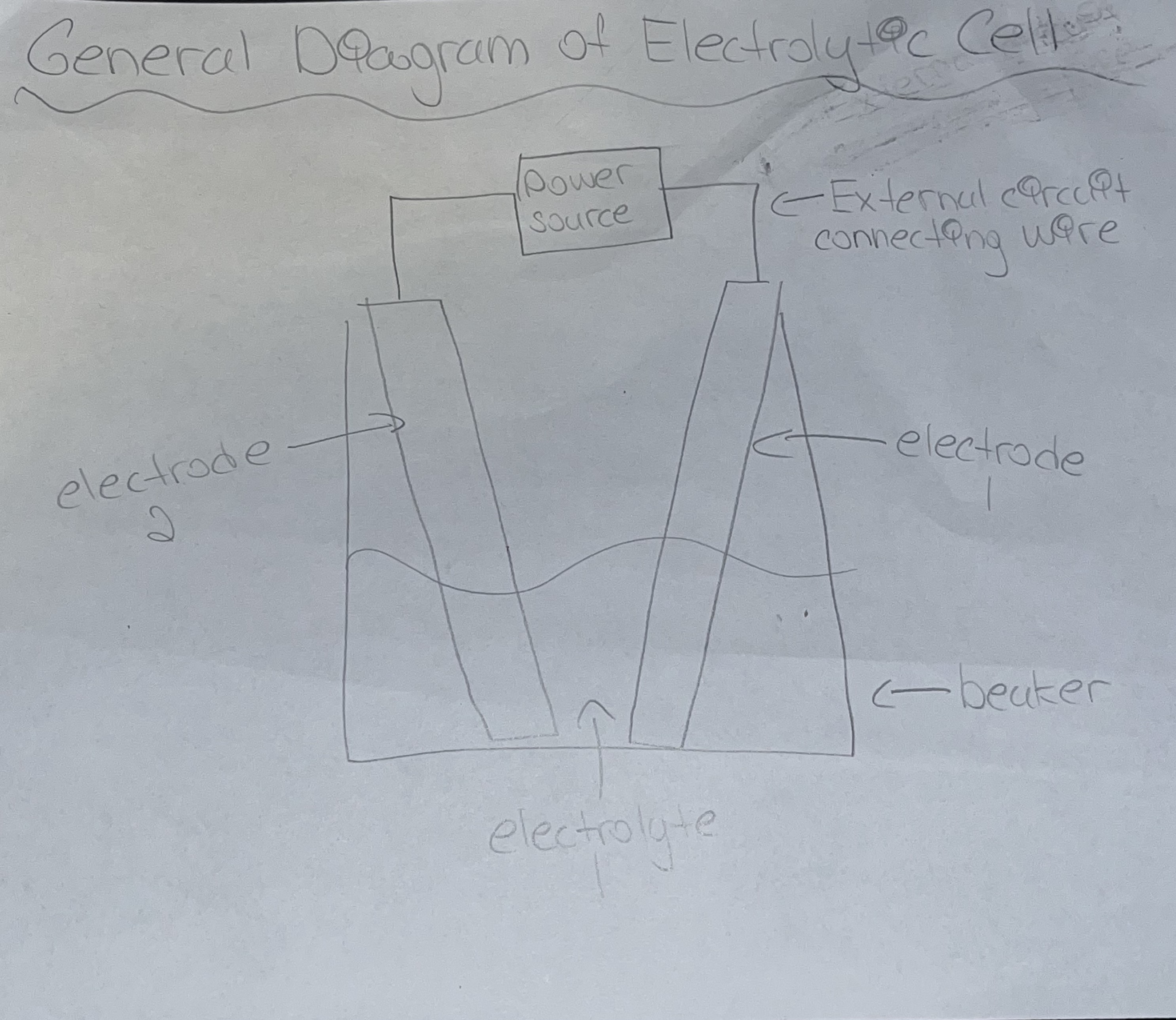
Voltaic Cell
Anode to cathode
Spontaneous
Anions = Anode = SRA = Oxidation
Cations = Cathode = SOA = reduction
Positive standard cell potential
Porous boundary
Produces electricity
Electrolytic Cell
Anions = Anode = SRA = Oxidation
Cations = Cathode = SOA = reduction
Anode to cathode
Non spontaneous
Negative standard cell potential
Non porous boundary
Requires electricity
Chloride Anomaly
When chloride ions and water are the only RA’S the chlorine becomes the SRA despite water being the SRA
Chlorine gas will be formed
Pure Copper
Cathode
Impure Copper
Anode