Chemistry unit 4 (Originally: Chemistry lab quiz/properties of bonds)
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
Ionic bonds
forms when a metal loses an electron and gives it to a nonmetal
What shape do ions take when they form?
Crystal lattice structure
How is a crystal lattice structure made?
The negative anions are surrounded by the positive cations. Energy is released when the bonds are formed. When larger amounts of energy are released the bonds are stronger and more stable.
Ionic bonds properties
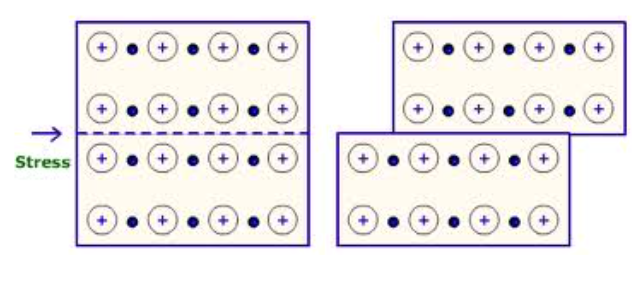
High melting and boiling points: cations are strongly attracted to anions on all sides so it takes a large amount of energy to break the numerous bonds
Hardness: ions in the crystal are locked in place by their bonds so the structure is very rigid and forms hard solids
Brittle (not malleable or ductile): shifting ions in the crystal can cause similar charges to line up next to each other, repulsion forces the crystal to fall apart
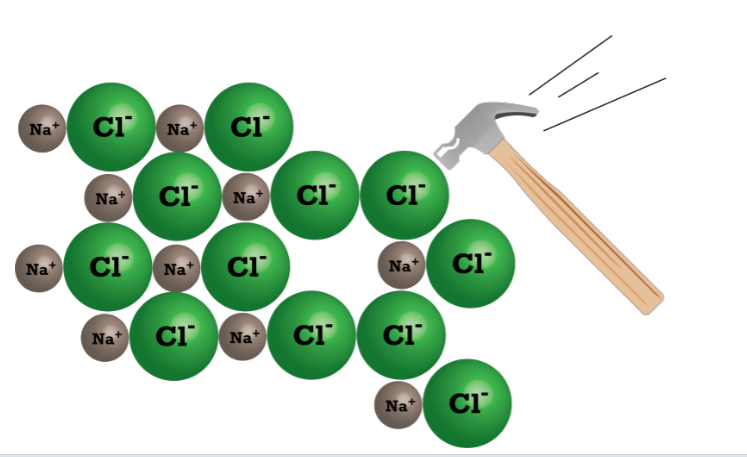
More ionic bond properties
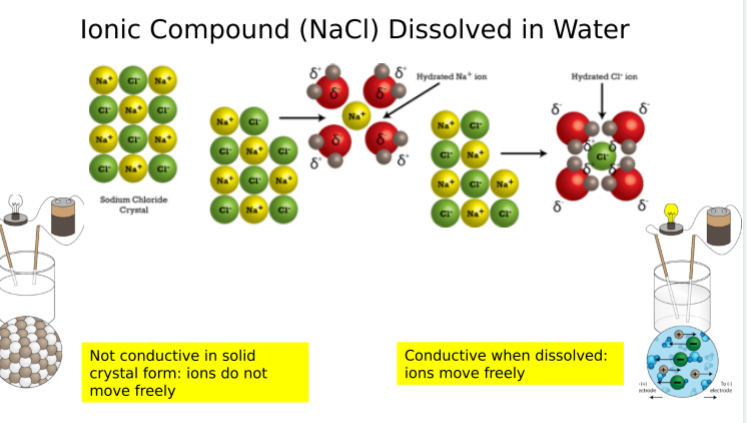
Ionic compounds do not conduct electricity when they are in solid form because the ions cannot move freely. However, when dissolved (or melted), ions are separated from each other and free to move. This allows an electrical current to flow easily through the metal or solution.
Covalent bonding
In covalent bonding, electrons are shared to complete valence shells (usually octets)
Covalent bonding usually occurs between two nonmetals
Compounds that contain covalent bonds are called molecules
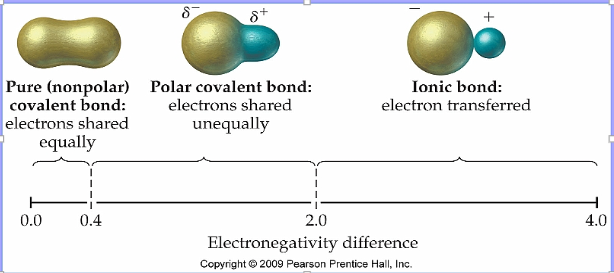
Covalent bonding and polarity
Polarity: unequal sharing of electrons in a covalent bond
Determined by differences in electronegativity
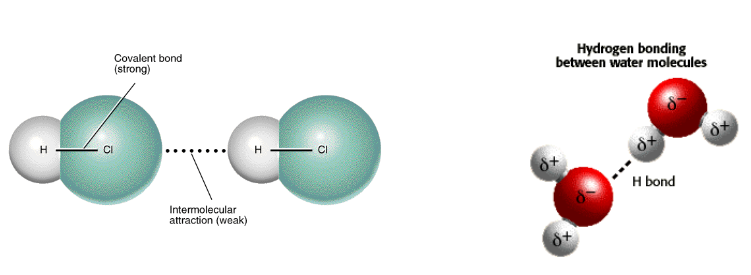
Intermolecular forces
Intermolecular Forces: weak attractions between individual molecules
Weaker than covalent bonds (and easier to break)
Only forces that hold molecules together in the liquid and solid states
These forces are what gives covalent bonds their properties, not the covalent bond itself
What happens when a covalent bond is dissolved in water?
The molecule is still not conductive; even when dissolved, no free-floating ions are produced
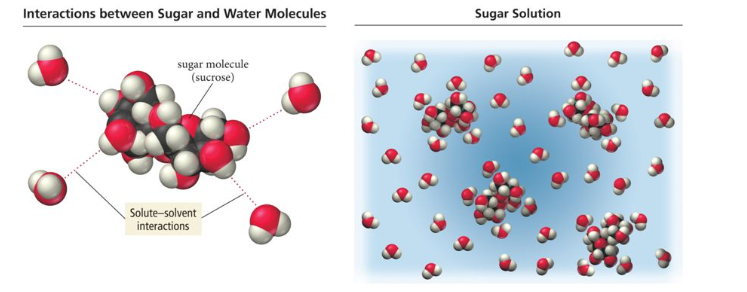
Covalent vs ionic bonds when dissolved in water, and how that effects their ability to conduct electricity (example uses salt and sugar, sugar being the covalent and salt being ionic)
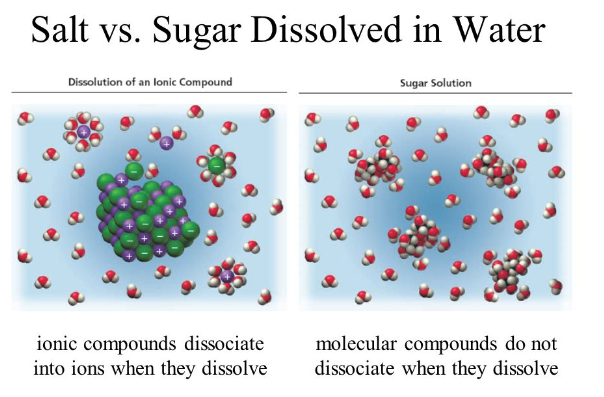
Covalent properties
Low melting and boiling points (compared to ionic compounds)
Molecules only held together by weak inter molecular forces
Many molecules are gases at room temperature
Low hardness (covalent compounds tend to be soft)
Weak inter molecular forces allow molecules to move easily (not locked tightly into place like ionic compounds)
Limited solubility in water
Water is polar and can only attach to and dissolve substances that are also polar or charged (ionic)
Many molecules are non-polar, so they don’t dissolve
Poor conductors of electricity
Freely-moving ions needed to carry an electrical current
Many molecules do not separate into ions in water
Metallic bonding
Valence electrons are held weakly by the nucleus, and can easily move from atom to atom. Electrons are said to be delocalized and from a “sea of mobile electrons.” Strength of bonds determined by heat of vaporization.
Heat of vaporization
Heat of Vaporization: amount of energy required to turn a metal (usually a solid) into a gas
positive energy values since it measures the energy required to break bonds
Metallic Bonding: Conductivity, MP and BP
High melting points (mp) and boiling points (bp): the positive nuclei are strongly attracted to many delocalized electrons surrounding it, so large amounts of energy needed to break bond
Conductivity: outer electrons move freely through a metal making them good conductors of heat and electricity.
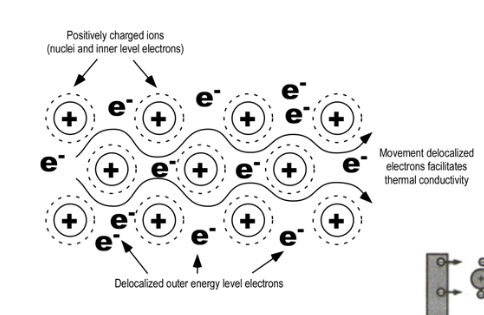
Metallic Bonding: Malleability & Ductility
Malleable & Ductile: metals can be hammered into sheets or rolled into wires easily. The positive nuclei simply shift past one another through the sea of electrons, but do not lose their attraction to the electrons.
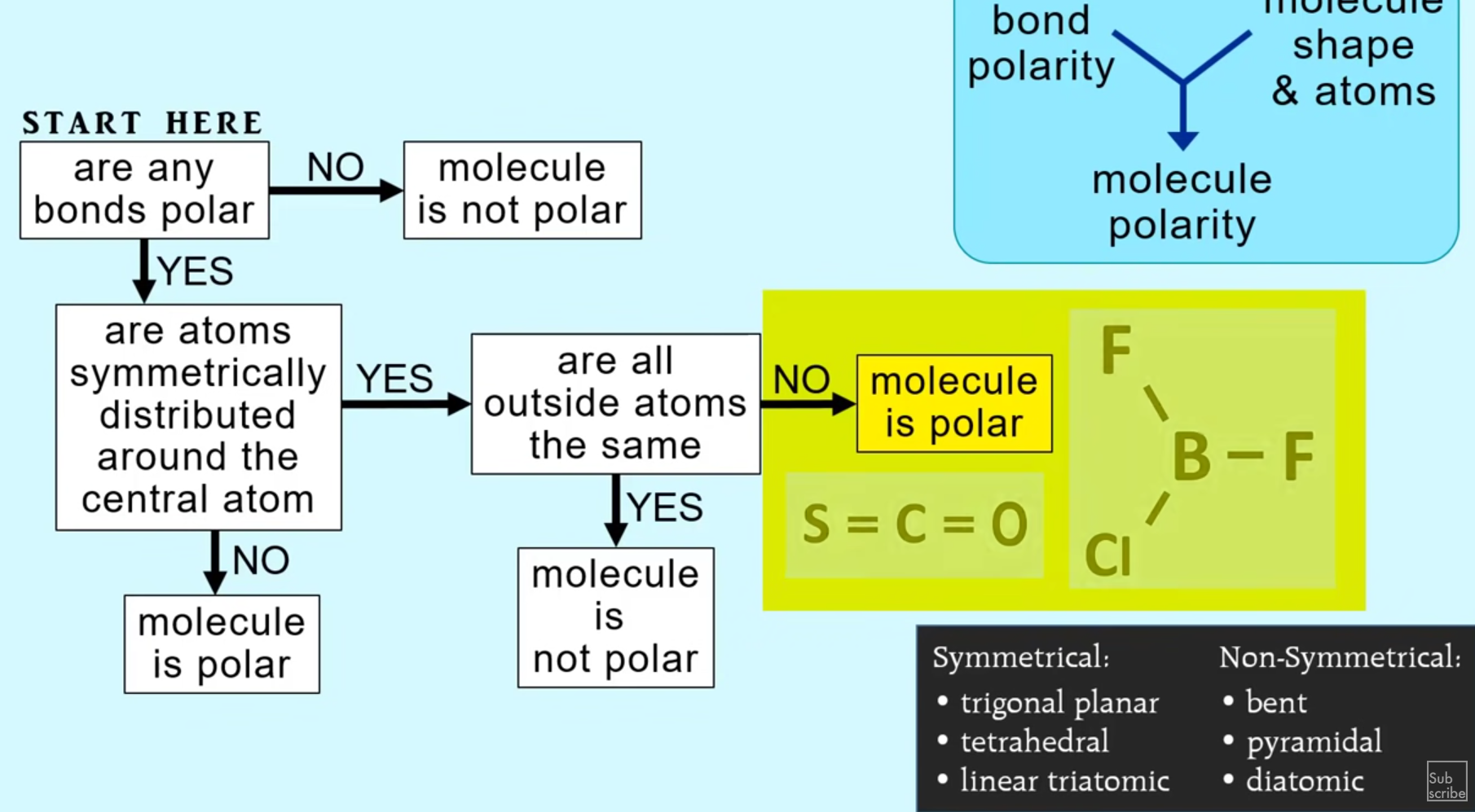
How to determine polarity of a molecule
Look at electronegativity
Look at the symmetry, if a molecule is symmetrical and has the same element surrounding the central atom, it must be non-polar
If a molecule is not symmetrical, it must be polar because there are some electrons somewhere (lone pairs) pushing the shape into a non-symmetrical shape
Why are the angles in a trigonal pyramidal smaller than the angles of a tetrahedral
Because the trigonal pyramidal is basically a tetrahedral but the top atom is replaced by a lone pair, which pushes on the atoms surrounding the central atoms a little more than an atom would, creating smaller angles (same goes for trigonal planar and bent). The electrons on the outside of the central atom just takes up more space.
What VSEPR shapes have lone pairs on the central atom?
Trigonal pyramidal and bent
What VSEPR shapes have no lone pairs on the central atoms?
Tetrahedral, linear, and trigonal planar
How to determine the number of bonds in a lewis structure?
add the max number of valence electrons each element can have (8 for most, 2 for hydrogen)
Add the number of valence electrons that each element has (adjust for ions)
#1-#2 (max number-current number)
divide the number you get by 2
this gives you the number of bonds you will have
Acid nomenclature for binary acid
Binary Acid
Hydrogen + nonmetal (monoatomic ion)
Change the –ide ending of the anion to -ic
Add the prefix hydro-
Examples:
HCl – hydrochloric acid
chloride became chloric
Hydro- prefix added
Acid nomenclature for an oxyacid (mostly polyatomics)
Oxyacids (Ternary Acids)
Hydrogen + nonmetals (polyatomic ion)
Anions with –ate endings change to -ic
Anions with –ite endings change to –ous
Do Not Add Hydro-
Examples:
HClO3 –chloric acid
chlorate became chloric
Covalent nomenclature
First element keeps its regular name
Second element gets –ide ending
Add numerical prefixes to both elements
Mono- only used on second element
Prefixes ending in “a” or “o” drop the last vowel if it’s followed by another vowel
Examples:
CO – carbon monoxide
oxygen became oxide
Mono- became Mon- before oxide
S block metal and nonmetal (fixed charge metals)
metal retains its name
nonmetal retains its ionic name (-ide)
ex: Sodium chloride, Calcium chloride (CaCl2)
Variable charge metal +nonmetal
Metal get its charge written with roman number in () after name
nonmetal retains its ionic name
Variable charge metals are transition metals, d block, and elements under the staircase
Ex: Fe+3 + Cl- =iron(III) chloride
Lattice energy
Energy being released when bonds form (sometimes given in negative but just take absolute value to find strongest bonds)
Bond energy
Energy added to break bonds, number should be positive
Bond length and bond energy
Bond length decreases as you gain bonds, and as bond energy increases, attraction is stronger, so bond length goes down
Ammonium
(NH4)+
Hydroxide
(OH)-
Carbonate
(CO3)-2
Nitrate
(NO3)-
Phosphate
(PO4)-3
Sulfate
(SO4)-2
Acronym to remember polyatomics you need to memorize
Nick the Camel ate Supper in Phoenix
(Use the first letter to give the name of the polyatomic, count the number of consonants to give the number of O’s, and count the vowels to give the charge)
Ex Nitrate→ 3 consonants and 1 vowel in Nick → (NO3)-
examples this acronym doesn’t cover that you still need to know: Ammonium (NH4)+ and hydroxide (OH)-
What random elements look like they should have a roman numeral but instead have a fixed charge?
Al has a fixed charge of +3, Zn has a fixed charge of +2, and Ag has a fixed charge of +1