topic 9 - separate chemistry 2
1/117
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
118 Terms
flame test - flame colour of a potassium ion (K+)
lilac
flame test - flame colour of a sodium ion (Na+)
yellow
flame test - flame colour of a lithium ion (Li+)
red
describe the test to identify halide ions
add dilute nitric acid to unknown solution
add silver nitrate to unknown solution
observe precipitate colour produced
results for test to identify halide ions (Cl-, Br-, I-)
chloride ion (Cl-) = white precipitate
bromide ion (Br-) = cream precipitate
iodide ion (I-) = yellow precipitate
describe the test to identify carbonate ions
add dilute hydrochloric acid
observe whether bubbling occurs
results for test to identify carbonate ions
if bubbling occurs, carbonate ions are present
explain why the results of a test for presence of specific ions must be unique
in order to identify the specific ion without uncertainty
what is the precipitation colour of an aluminium ion (Al3+)
white (dissolves and goes colourless when excess NaOH is added)
describe the test to identify ions in solids or solutions using sodium hydroxide solution (NaOH)
add NaOH solution to unknown solution
observe the precipitate colour produced
if a white precipitate is formed add excess NaOH solution
flame test - flame colour of a copper ion (Cu2+)
blue-green
flame test - flame colour of a calcium ion (Ca2+)
brick red (orange-red)
precipitate test - precipitate colour of a iron 2 ion (Fe2+)
green
precipitate test - precipitate colour of a copper ion (Cu2+)
blue
precipitate test - precipitate colour of a calcium ion (Ca2+)
white
precipitate test - precipitate colour of a ammonium ion (NH4+)
white
precipitate test - precipitate colour of a iron 3 ion (Fe3+)
brown
describe the test to identify sulfate ions
add dilute hydrochloric acid to unknown solution
add barium chloride to unknown solution
observe whether a white precipitate is formed
results for test to identify sulfate ions
if a white precipitate is formed, sulfate ions are present
describe the flame test to identify ions in solids
dip flame test loop into dilute hydrochloric acid
hold flame test loop in flame and then dip in beaker of water
dip clean flame test loop into one of the four known solids
observe and record the flame colour produced
describe the test for hydrogen gas
place a lighted splint in a test tube containing the gas. if the gas is hydrogen it will produce a squeaky pop
describe the test for oxygen
place a glowing splint in a test tube containing the gas. if oxygen is present the splint will relight
describe the test for carbon dioxide
bubble the unknown solution through limewater. if carbon dioxide is present, a white precipitate of calcium carbonate will be formed
describe the test for ammonia gas
dip a glass rod in concentrated hydrochloric acid and put this in the unknown gas. if ammonia is present, a white smoke of ammonium chloride will form
describe the test for chlorine gas
place a damp piece of litmus paper above a test tube containing the unknown gas. if the litmus paper is bleached, chlorine is present
what are the advantages of instrumental methods of analysis
they are readily available
can improve sensitivity of test
can improve accuracy of test
can improve speed of test
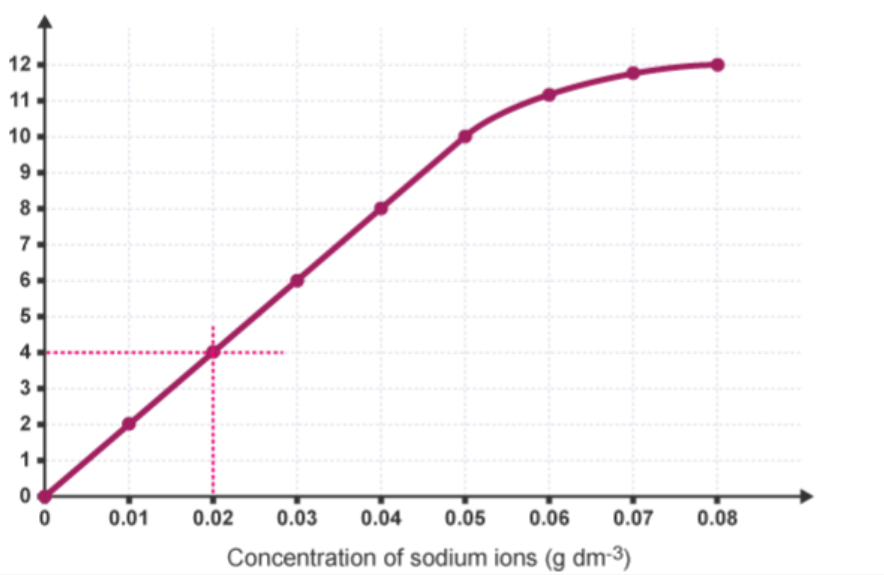
explain how to determine the concentration of ions in a dilute solution using a calibration curve
plot a calibration graph using concentration data points and flame photometer readings
y-axis is flame photometer readings
x-axis is concentration of ions
draw a horizontal line to the calibration curve from the chosen point on the y-axis
from the point the horizontal line connects to the curve, draw a vertical line down to the x-axis
work out the concentration of ions based on where the vertical line touches the x-axis
explain how to identify metal ions by comparing data with reference flame photometer data
use a flame photometer to split the coloured light from a vaporised sample into an emission spectrum
compare this spectrum to the reference flame photometry spectrum
identify the metal ion from the reference spectrum
recall the molecular formulae of methane
CH4
recall the molecular formulae of ethane
C2H6
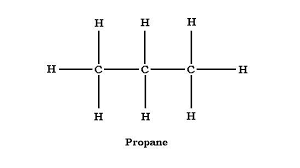
recall the molecular formulae of propane
C3H8
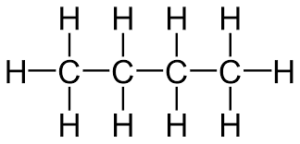
recall the molecular formulae of butane
C4H10
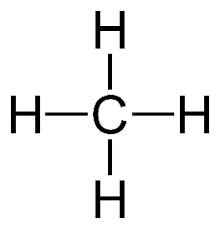
recall the displayed structure of methane
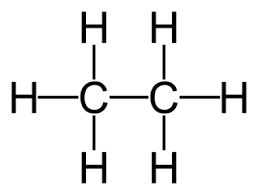
recall the displayed structure of ethane
recall the displayed structure of propane
recall the displayed structure of butane
explain why alkanes are saturated hydrocarbons
compound that contains ONLY carbon and hydrogen atoms
and contains only single bonds
between carbon atoms
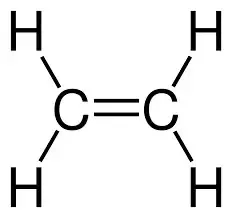
recall the molecular formulae of ethene
C2H4
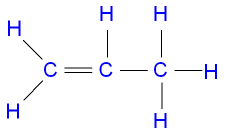
recall the molecular formulae of propene
C3H6
recall the molecular formulae of butene
C4H8
recall the displayed structure of ethene
recall the displayed structure of propene
recall the displayed structure of but-1-ene
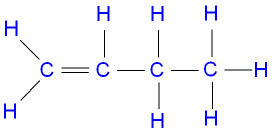
recall the displayed structure of but-2-ene
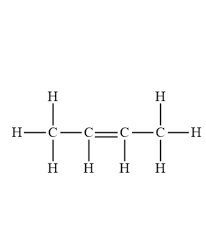
explain why alkenes are unsaturated hydrocarbons
compound that contains ONLY hydrogen and carbon atoms
and contains at least 1 double bond between carbon atoms
recall what occurs in the addition reaction between ethene and bromine
ethene + bromine → 1, 2-dibromoethane
double bond between carbon atoms in ethene breaks
to connect to the 2 bromine atoms
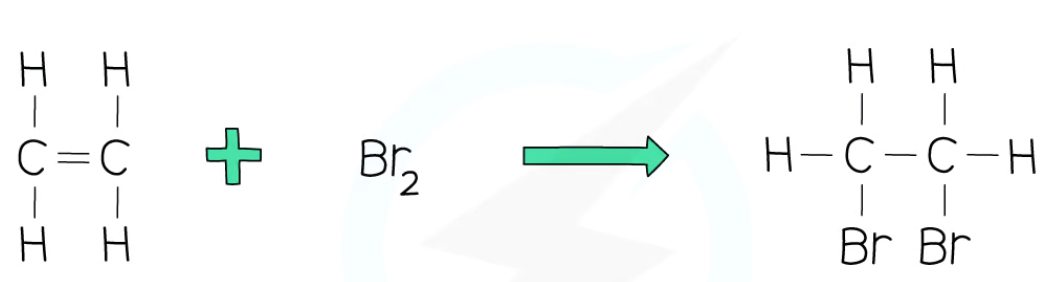
explain how bromine water can be used to determine between alkanes and alkenes
alkanes - the orange bromine water will remain orange
alkenes - the orange bromine water will become colourless
because if an unsaturated compound is shaken with the bromine water, an addition reaction will occur
causing the orange solution to turn colourless
state what the complete combustion of alkanes/alkenes involves
involves oxidation of hydrocarbons
to produce carbon dioxide
and water
state what a polymer is
substance of high average relative molecular mass
consisting of small repeating units
explain how ethene molecules combine together in a polymerisation reaction
one of the bonds in the carbon double bond in ethene breaks
this allows the ethene monomer to bond to other ethene monomers
they create a long, repeating chain of ethene monomers
to create a poly(ethene) polymer
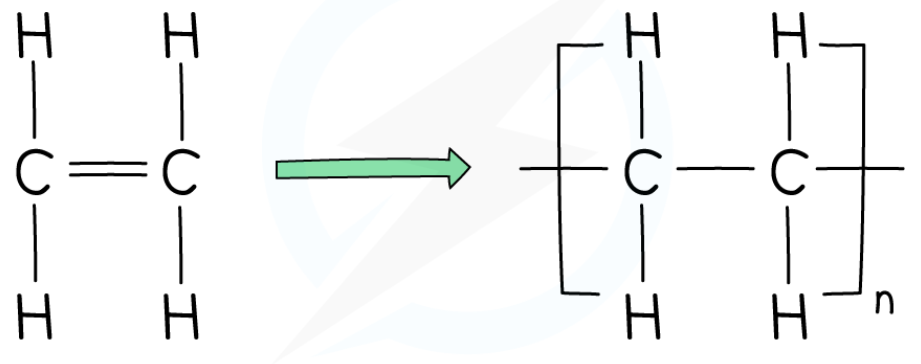
state what the product of the polymerisation of ethene is
poly(ethene)
explain the polymerisation reaction of atoms to create poly(propene)
one of the bonds in the double bond between carbon atoms in propene breaks
this allows the propene monomer to bond to other propene monomers
they create a long, repeating chains of propene monomers
called a poly(propene) polymer
state what the product of the polymerisation of propene is
poly(propene)
explain the polymerisation reaction of compounds to create poly(chloroethene)
one of the bonds in the double bond between the carbon atoms in ethene breaks
this allows the carbon atoms in ethene to bond to the one chloride atom
this creates chloroethene
chloroethene monomers bond together
to create long, repeating chain of chloroethene
called poly(chloroethene)
explain the polymerisation reaction of compounds to create poly(tetrafluoroethene)
one of the bonds in the double bond between the carbon atoms in ethene breaks
this allows the carbon atoms in ethene to bond to the four fluorene atoms
creating tetrafluoroethene
tetrafluoroethene monomers bond together
to create a long, repeating chain of tetrafluoroethene
called poly(tetrafluoroethene)
explain how to deduce the structure of a monomer from the structure of an addition polymer
identify the repeating unit in the polymer
change the single bond between carbon atoms in the repeating unit
to a double bond in the monomer
remove the bond from each end of the repeat unit and the subscript n
explain how the use of poly(ethene) in carrier bags is related to its properties
thin
cheap
can be made into thin film
explain how the use of poly(ethene) in food wrap is related to its properties
can be made into thin film
cheap
flexible
explain how the use of poly(ethene) in shampoo bottles is related to its properties
cheap
explain how the use of poly(propene) in buckets is related to its properties
strong
resists shattering
explain how the use of poly(propene) in bowls is related to its properties
strong
resists shattering
explain how the use of poly(propene) in crates is related to its properties
strong
resists shattering
explain how the use of poly(propene) in ropes is related to its properties
flexible
strong
explain how the use of poly(chloroethene) in insulation for electrical wires is related to its properties
electrical insulator
flexible
explain how the use of poly(chloroethene) in windows is related to its properties
tough
explain how the use of poly(chloroethene) in gutters is related to its properties
tough
explain how the use of poly(chloroethene) in pipes is related to its properties
tough
hard
explain how the use of poly(tetrafluoroethene) in non-stick coatings for pans is related to its properties
slippery
chemically unreactive
explain how the use of poly(tetrafluoroethene) in containers for laboratory substances is related to its properties
chemically unreactive
explain what a condensation polymer is
formed when two different monomers are linked together
with the removal of a small molecule
usually water
state what an ester link is
a link formed by a condensation reaction
between an alcohol
and a carboxylic acid
explain why polyesters are condensation polymers
polyester is formed when a monomer molecule containing 2 carboxylic acid groups
is reacted with a monomer molecule containing 2 alcohol groups
one molecule of water is formed every time an ester link is formed
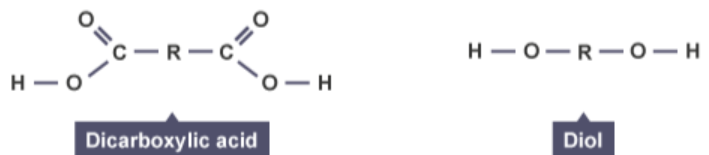
explain why the availability of starting material is an issue associated with polymers
there is a low availability of starting material
making the creation of polymers a longer and more expensive process
explain why the persistence of polymers in landfill sites is an issue associated with them
polymers are non-biodegradable
meaning when they are placed in landfill sites, they will not be broken down by microorganisms
causing polymers to remain in landfill sites for long periods of time
and waste space in landfill sites
explain why gases produced during disposal by combustion is an issue associated with polymers
when polymers are burnt, carbon dioxide is produced
carbon dioxide has a negative effect on the environment as it enhances the greenhouse effect
in enclosed spaces, carbon monoxide can also be produced
which can suffocate humans as it deprives the body of oxygen
explain why the requirement of sorting polymers for recycling is an issue associated with polymers
separating different polymers is difficult
and expensive
state the advantages of recycling polymers
polymers are finite resources, meaning recycling prolongs their supply
reduces the amount of non-biodegradable polymers ending up in landfill
reduces the amount of crude oil needed as crude oil is the raw material needed to make new polymers
prevents disposal of polymers by combustion, reducing the amount of carbon dioxide emissions produced
re
state the disadvantages of recycling polymers
recycling polymers is difficult
recycling polymers is expensive
recycling process is energy-intensive which can contribute to climate change
recycled polymers requires melting, which can produce toxic gases harmful to animals and plants
recycling runs the risk of mixing different polymers together, which can alter the properties of the polymers
recall what DNA is
a polymer
made from four different monomers
called nucleotides
recall what starch is
a polymer
based on sugars
recall what proteins are
polymers
based on amino acids
state the molecular formulae of ethanol
CH3CH2OH
state the molecular formulae of methanol
CH3OH
state the molecular formulae of propan-1-ol
CH3CH2CH2OH
state the molecular formulae of butan-1-ol
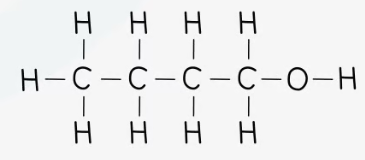
CH3CH2CH2CH2OH
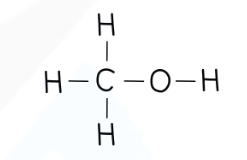
recall the displayed structure of methanol
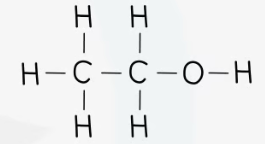
recall the displayed structure of ethanol
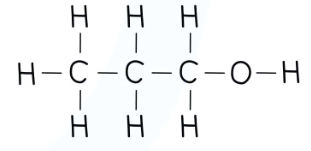
recall the displayed structure of propan-1-ol
recall the displayed structure of butan-1-ol
state what the functional group in alcohol is
-OH
state what is produced when alcohols are dehydrated
alkanes
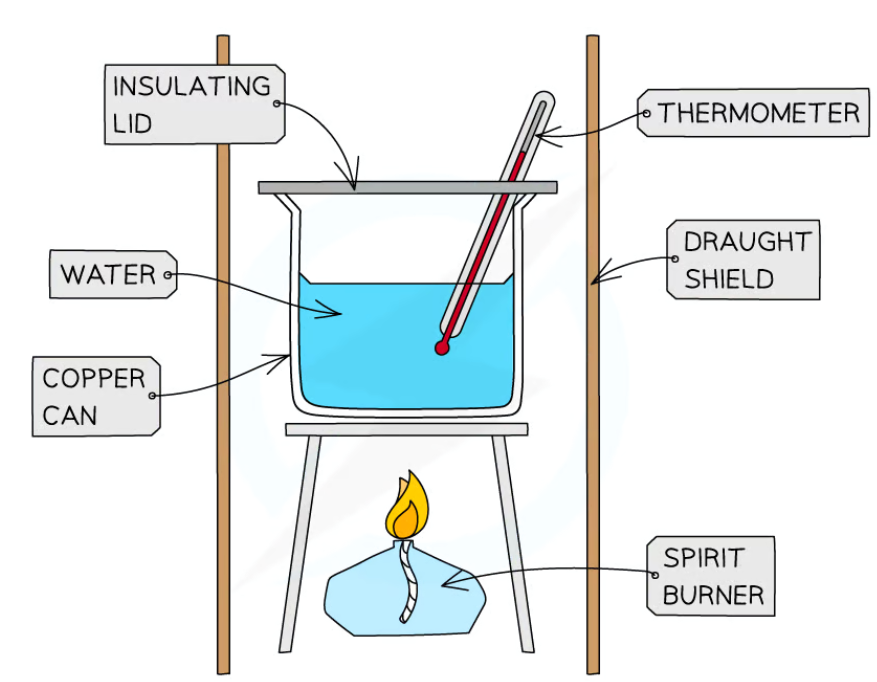
state the method of how to investigate the temperature rise in a known mass of water by the combustion of alcohols
set up a simple combustion calorimeter including a draught shield, insulating lid, thermometer and spirit burner
use a measuring cylinder to measure 100cm3 of water into a copper can
record the initial temperature of the water and the mass of the empty burner
fill the spirit burner with ethanol and record its new mass
place the spirit burner under the copper can, light the wick and place the insulating lid on
stir the water constantly with the thermometer
continue heating until the temperature rises by 25 degrees C
immediately extinguish the flame and record the final mass of the spirit burner
repeat these steps with propanol, butanol and pentanol, making sure to keep the volume of water and the distance between the wick and the bottom of the stand the same
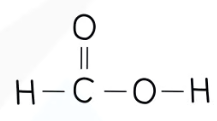
state the molecular formulae of methanoic acid
HCOOH
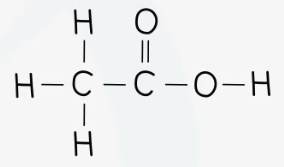
state the molecular formulae of ethanoic acid
CH3COOH
state the molecular formulae of propanoic acid
CH3CH2COOH
state the molecular formulae of butanoic acid
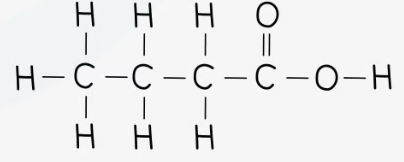
CH3CH2CH2COOH
recall the displayed structure of methanoic acid
recall the displayed structure of ethanoic acid
recall the displayed structure of propanoic acid
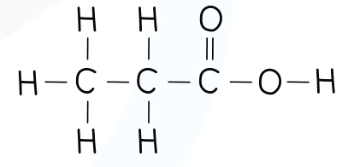
recall the displayed structure of butanoic acid