3-5 Soil solid phase
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
53 Terms
Organic matter in soil: C cycle
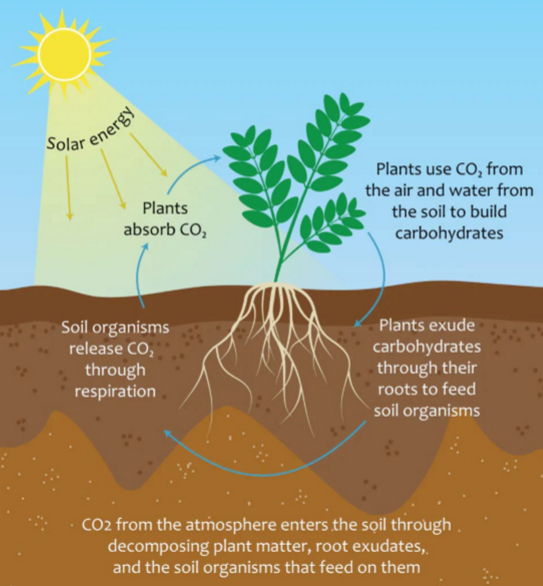
Soil one of the most important C reserve on earth
Nitrogen important for soil, organism in the soil can catch it in the atmosphere and break it for the soil → fertilizer are Nitrogen already broken for the soil
OM → Source of C and energy
Organic matter composition
Composition of the OM: C (50%), H, O, N, S, P and metal cations
Changes from soil to soil, from season to season…
Content of OM in the topsoil:

Concentration vs Stock of organic C
Concentration → Indica quanto carbonio c’è nel suolo in proporzione alla massa del suolo. → in % o g C / kg di suolo
Stock → Indica quanto carbonio totale è contenuto in un certo volume o area di suolo. → kg C / m²
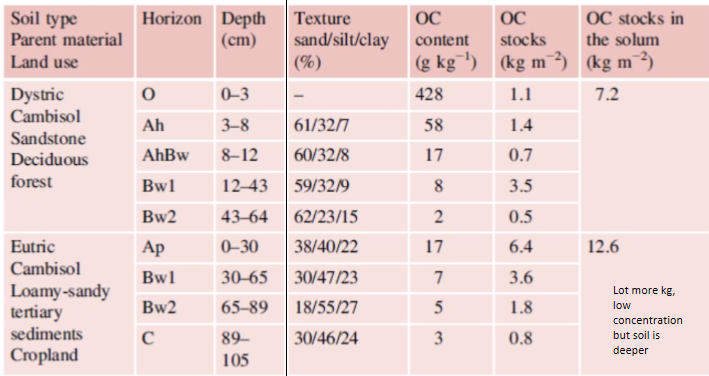
Content*(density or area) → stock
Concentration is important but stock also
Global stock C distribution
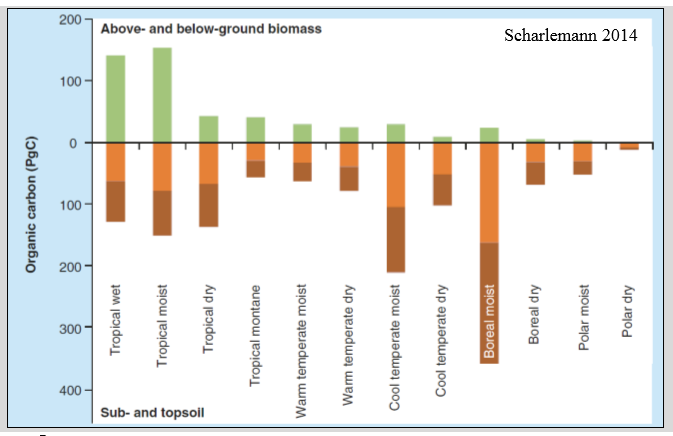
More in the soil than in plants, more in colder places cause there are less organism
Soil OM: global fluxes and stocks

OM in soil definitions
Organic matter = humus in the broad sense: all dead plant and dead animal substances in and on the mineral soil and their organic transformation products
Edaphon (soil biota): living organisms and roots → not part of the soil organic matter
Mineralization: complete microbial degradation to inorganic substances
Humification: formation of humus = protection of OM from further decomposition
Decomposition: breakdown of organic matter → transformation of organic residues by heterotrophic organisms leads to differentiation of the organic matter:
Plant remains
Microbial remains
Mineral-bound organic substance
Charcoal
Dissolved organic carbon (DOC)
C/N Ratio
Indicator for the ease of decomposition of OM but also for biological activity
Mineralisation = release of CO2 while N is incorporated into microbial biomass → C/N becomes smaller → C/N ratio goes lower when you lose carbon, because Nitrogen doesn't get lost
Degradability of organic matter: the smaller the more degradable → spruce woods really degradable
Composition of OM: Plant remains
Parenchyma (basic tissue)
Found in living green tissues (leaves, needles, fine roots)
Cell walls mainly contain cellulose and hemicellulose
Rich in proteins
Low C/N ratio (< 50)
Easily degradable
Lignified tissue
Includes wood (xylem) and supporting tissue (sclerenchyma)
Cell walls contain cellulose, hemicellulose, and lignin
High C/N ratio (> 100)
Structurally strong and resistant to decomposition
Cellulose
Main structural component of plant cell walls
Long chains of glucose forming strong microfibrils
Mostly crystalline, partly amorphous
Provides mechanical strength
Usually associated with hemicellulose and lignin
Hemicellulose
Cell wall polysaccharides made of different sugars
Shorter and more branched than cellulose
Amorphous and easily degradable
Links cellulose microfibrils together
Lignin
Very complex and highly cross-linked polymer
Common in vascular and woody plant tissues
Provides rigidity, protection, and resistance to microbes
Very resistant to degradation (not easily broken down)
Composition of OM: Plant wax and fats
Lipids
Mostly unbranched hydrocarbon chains
Chemically diverse group (fatty acids, phospholipids, glycolipids)
Key components of biological membranes
Important for energy storage and waterproofing
Generally more resistant to degradation than carbohydrates
Cutin
Structural polymer forming the plant cuticle (wax layer)
Found on the surface of leaves and stems
Built from hydroxy fatty acids
Provides protection against water loss and pathogens
Contributes to plant resistance and durability
Suberin
Cell wall component in outer tissues of woody plants
Especially abundant in bark and roots
Similar to cutin but made of longer-chain components
Acts as a strong barrier to water, gases, and microbes
Very resistant to biological degradation
Composition of OM: cellular constituent
Proteins
Long chains of amino acids (polypeptides)
Present in plant and microbial tissues
Readily degradable by many microorganisms
Important nutrient source (especially nitrogen)
Less stable than structural compounds like lignin
Tannins
Polyphenolic compounds
Bind to proteins and reduce their availability
Can slow down microbial decomposition
Common in leaves, bark, and woody tissues
Other Cellular Compounds
Pigments: e.g. chlorophyll
Starch: energy storage compound in plants
Nitrogen, sulphur and phosphorus in OM
Nitrogen → Almost exclusively binding to OM in soil (95%) → First in microbial biomass, then stabilized in OM → Mostly in peptides
Sulfur: → up to 90% S in organic form, 30 - 75% as organo sulphates, rest as amino acids
Phosphorus → > 50% bound in the form of orthophosphate
Mineralization and humification
Mineralization
Microbial breakdown of organic matter into inorganic nutrients
Produces carbon dioxide, water, and plant-available nutrients
Fast for easily degradable compounds (carbohydrates, proteins)
Leads to nutrient release into soil solution
Humification
Transformation of organic residues into stable humic substances
Favored by resistant compounds (lignin, fats, waxes, tannins)
Slower process than mineralization
Contributes to long-term carbon storage in soils
Decomposition of litter
Plant litter is decomposed by microorganisms and soil fauna
Early phase: biochemical breakdown of simple compounds
Initial phase: leaching and rapid microbial growth
Crushing phase: mechanical fragmentation by soil fauna
Final phase: enzymatic degradation, nutrient release, accumulation of resistant compounds
Key idea: Litter decomposition drives nutrient cycling and soil organic matter formation.
Biochemistry of degradation processes
Biochemistry of Degradation Processes
Easily degradable polymers are enzymatically hydrolyzed early
Proteins, starch, nucleic acids, simple lipids
Complex and resistant compounds require prior physical breakdown
Cellulose: degraded by specialized enzyme systems
Lignin: broken down via oxidative reactions
End result: small molecules that serve as microbial food
Speed of litter degradation
Litter factors (degradability):
N content of the substance (C/N ratio)
Lignin content (lignin/N ratio)
Tannin content (polyphenol/N ratio)
External factors, environmental factors:
Heat/temperature
Availability of H2O and O2
pH
Inhibitors (bactericides, fungicides)
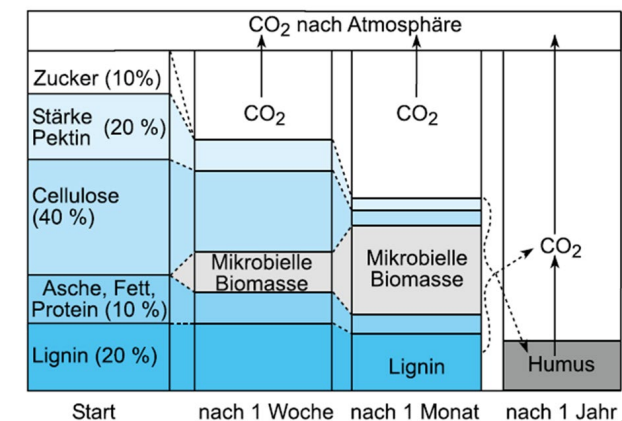
Stabilisation of OM
Stabilisation (Humification)
Processes that slow down organic matter decomposition
Leads to accumulation of humus in soil
Stabilized organic matter is older and more persistent
Stabilisation by Recalcitrance
Delayed degradation due to molecular structure
Primary recalcitrance: plant-derived compounds with resistant structures (litter, roots)
Secondary recalcitrance: microbial and animal products forming resistant macromolecules
Includes pyrogenic (black) carbon, highly resistant
Key Idea → Chemical complexity increases resistance to microbial breakdown
Old and new concept of stabilisation
Old Concept
Soil organic matter forms large, stable humic macromolecules
Stabilisation driven mainly by chemical condensation reactions
Humic substances (humic acids, fulvic acids, humin) seen as real soil entities
Molecular complexity = long-term stability
New Concept
Soil organic matter is mostly made of small, simple biomolecules
Stability depends on environmental protection, not molecular size
Key mechanisms: mineral association, physical protection, limited microbial access
Carbon persistence is dynamic and can change with conditions
Key Idea → Soil carbon is stabilized by its environment, not by intrinsic “humic” polymers
Stabilization through sorption and aggregation
Stabilisation through Sorption
Organic matter binds to mineral surfaces (clays, metal oxides)
Sorption reduces accessibility for microbes and enzymes
Strong organo-mineral associations increase persistence
Particularly important for small organic molecules
Stabilisation through Aggregation (Physical Disconnection)
Organic matter is physically protected inside soil aggregates
Limited access of microorganisms, oxygen, and enzymes
Larger aggregates store carbon short- to medium-term
Mineral-associated organic matter enables long-term storage
Key Idea → Soil carbon stability is controlled by physical protection and mineral interactions, not by molecular complexity alone
Importance of organic matter for soil
Major nutrient source for plants (especially nitrogen, phosphorus, sulfur)
Improves nutrient retention and gradual release
Enhances soil aggregation and structural stability
Reduces erosion through clay–organic matter complexes
Increases water-holding capacity
Influences soil temperature via light absorption
Supports decomposition of organic pollutants
Key reservoir for carbon storage (e.g. peatlands, permafrost, biochar)
Key Idea: Soil organic matter is essential for soil fertility, structure, and climate regulation.
Humus forms
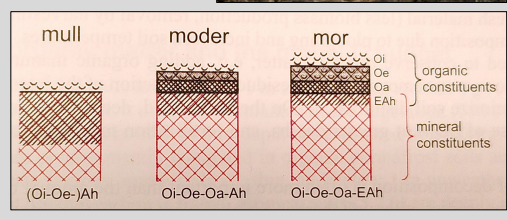
Mull
Most favorable humus form
Nutrient-rich soils
Neutral to slightly acidic
High microbial and soil fauna activity
Fast litter decomposition
No distinct organic (Oa) layer
Typical of deciduous forests and species-rich grasslands
Moder
Intermediate between Mull and Mor
Neutral to slightly acidic
Moist conditions
Moderate biological activity
Oi, Oe, and Oa horizons present with gradual boundaries
Common in coniferous, mixed, and deciduous forests
Mor
Least favorable humus form
Nutrient-poor soils
Acidic, often very wet or very dry
Low microbial and animal activity
Fungi-dominated decomposition
Thick litter layer, slow decomposition
Sharp boundaries between Oi, Oe, and Oa
Typical of coniferous forests and harsh environments
Key Idea:
Humus forms reflect decomposition intensity, biological activity, and nutrient availability.
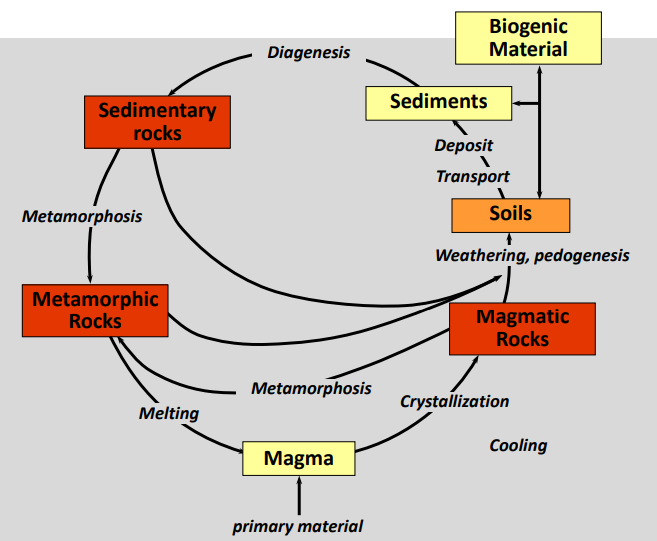
Parent material → Rocks
Soils are made of organic matter and rocks from the bedrock, also called parent material.
The parent rock of a soil largely determines the soil development and the mineral content.
Rocks are classified according to their history of origin: Magmatic, metamorphic and sedimentary
Magmatic rock soil properties:
Basalt / Gabbro → base-rich, nutrient-rich
Andesite / Diorite → intermediate
Rhyolite / Granite → silica-rich, nutrient-poor
Extrusive: fine-grained, chemical weathering dominant
Intrusive: coarse-grained, physical weathering dominant
Key idea: More silica → fewer bases → lower soil fertility.
Sedimentary rocks:
Clastic: formed from mechanical weathering (e.g. sandstone, shale)
Chemical: formed by precipitation from solution (e.g. rock salt, some limestones)
Organic: formed from accumulated biological material (e.g. coal, some limestones)
Key idea:
Sedimentary rocks form from weathering, precipitation, or biological accumulation.
Lithosphere cycle
Most common minerals in earth crust and soil
Most commons elements in earths crust → O and Si → SiO2 most common mineral
This is also true for Soils
Primary minerals → comes from the rock → broken down rock goes into the soil
Secondary minerals → chemical composition changes
Minerals: silicates
Origin
Main source of secondary (pedogenic) minerals
Formation depends on temperature, pressure, cooling rate, and chemistry
Crystallize in sequence from high to low temperature
Early: olivine, pyroxene
Late: feldspars, quartz
Silicate Structures
Isolated tetrahedra → olivine
Single chains → pyroxenes
Double chains → amphiboles
Sheets (layers) → micas, clay minerals
Framework → feldspars, quartz
Properties
Structures linked by oxygen bridges
Negative charge attracts metal cations
Isomorphic substitution creates chemical diversity
Structure controls stability and weatherability
Key Idea → More complex silicate structures are more stable and weather more slowly
Micas (sheet)
Types
Muscovite: light-colored mica
Biotite: dark-colored mica
Structure
Sheet (layer) silicates with a 2:1 structure
Layers held together by potassium ions
Importance for Soils
Important source of potassium
Easily weathered minerals
Biotite weathers faster than muscovite
Occurrence
Magmatic rocks: mainly biotite
Sedimentary and metamorphic rocks: mainly muscovite
Key Idea → Micas are nutrient-bearing, easily weatherable minerals important for soil fertility
Tectosilicate (3D) → Quartz and Feldspar
Quartz
Pure framework silicate
Very resistant to weathering
Common as sand and silt in soils and sediments
Does not supply nutrients
Hard, irregular fracture surfaces
Feldspars
Framework silicates with aluminum substitution
Charge balanced by potassium, sodium, or calcium
Most abundant minerals in magmatic rocks
Easily weathered compared to quartz
Smooth cleavage surfaces
Relevance for soil
Feldspars release nutrients during weathering
Weathering forms secondary (clay) minerals
Quartz accumulates due to high resistance
Key Idea → Quartz persists; feldspars transform and feed soils
Weathering
Weathering alters rocks and primary minerals
Occurs at the interface with atmosphere, biosphere, and hydrosphere
Transforms primary minerals into secondary minerals
Produces clay minerals, oxides, and hydroxides
Key process linking lithosphere to soil formation
Key idea:
Weathering is the bridge between rocks and soils.
Physicall weathering
Mechanical breakdown of rocks without chemical change
Reduces grain size and increases surface area
Main Processes
Pressure release: cracking after erosion of overlying rock
Gravity: impact and crushing
Temperature changes: differential expansion of minerals
Frost weathering: ice expansion widens cracks
Salt weathering: crystal growth in pores and fractures
Biological pressure: root growth
Transport & Abrasion
Water: rolling, abrasion, scouring
Wind: saltation and suspension of fine particles
Ice: transport and abrasion at glacier base
Key Idea:
Physical weathering fragments rocks and prepares material for chemical weathering and soil formation.
Chemical weathering
Chemical transformation or dissolution of minerals
Driven by water, acids, and biological activity
Continuous process because products are removed by water
Main Processes
Hydration
Water molecules enter mineral structures
Weakens crystal bonds and promotes dissolution
Especially important for highly soluble minerals (e.g. salts)
Hydrolysis / Protolysis
Reaction of minerals with acidic water
Carbonic acid from CO₂-rich water plays a key role
Very important for carbonate dissolution and silicate alteration
Complexation
Organic acids released during decomposition bind metal ions
Increases mobility of metals such as iron and aluminum
Enhances mineral dissolution and element transport
Oxidation
Reaction with O₂
Fe²⁺ → Fe³⁺, S²⁻ → SO₄²⁻
Produces H⁺, lowers pH
Forms Fe oxides/hydroxides (goethite, hematite)
Protolyis (Hydrolysis) of Silicates
Reaction of silicates with H⁺ (water / carbonic acid)
Breaks Si–O–Al bonds
Releases K⁺, Na⁺, Ca²⁺
Forms clay minerals (e.g. kaolinite) + silica
Weathering of Silicates – Overview
Early stage: loss of interlayer cations (e.g. K⁺), structure partly preserved
Advanced stage: complete lattice breakdown → new secondary minerals
Products: clays, oxides, hydroxides
Weathering Products
Weathering residues (solid remains)
Weathering solutions (dissolved ions transported away)
Key Idea → Chemical weathering transforms primary minerals and releases elements, driving soil formation and nutrient cycling.
Weathering stability of minerals
Water solubility → Easily soluble salts < gypsum < calcite < dolomite
Structure of the silicates → Island < chains < leaf < framework (feldspars < quartz)
Fe(II) content (oxidizability) → Biotite < Muscovite
In general → Olivine < Pyroxene < Amphibole < Biotite < Plagioclase < Muscovite ≈ Orthoclase < Quartz
Pedogenesis image
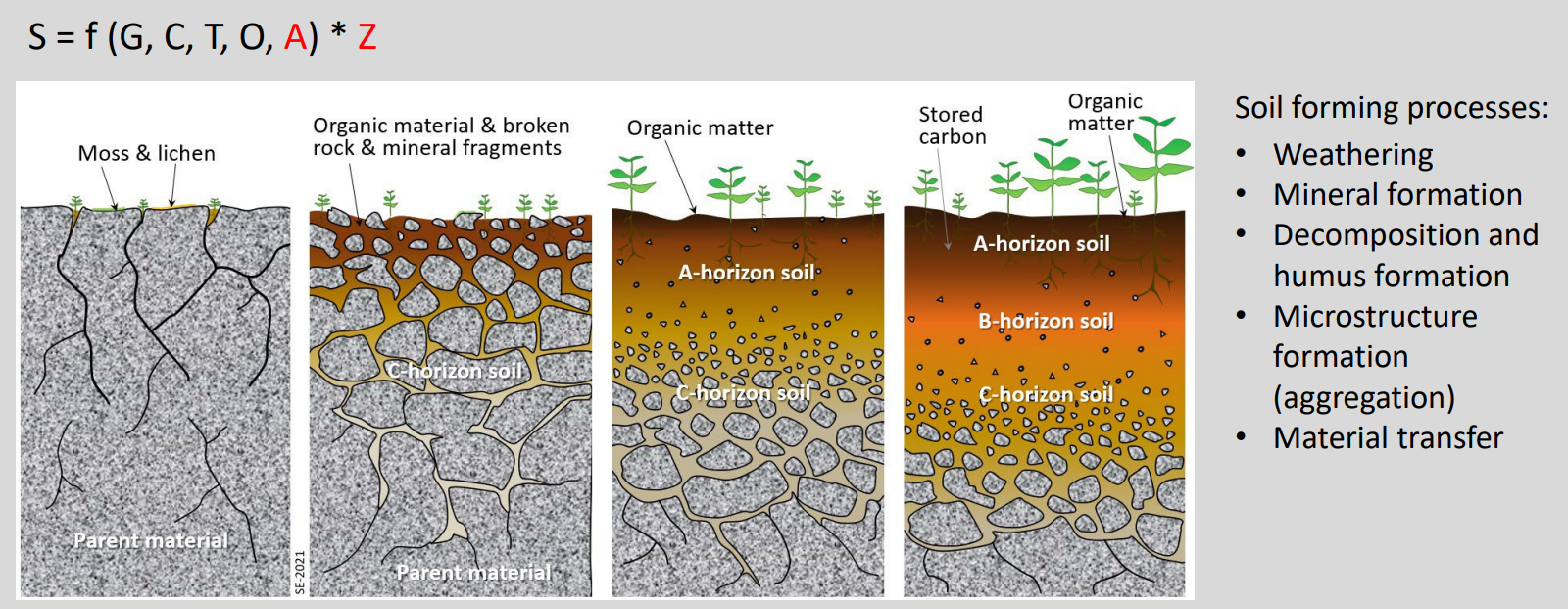
Secondary minerals
Formed after partial or complete dissolution of primary minerals in the parent material
Main products:
Clay minerals (from layer silicates, e.g. micas)
Fe, Mn and Al oxides and hydroxides
Secondary carbonates and salts
These secondary minerals form in soils during pedogenesis.
Clay Mineral
Origin
Formed from silicate weathering
Structure similar to mica (tetrahedral + octahedral layers)
Doesn’t change structure → Still tetrahedra/octahedra but with different minerals and charge
Particle size < 2 µm (clay fraction)
Identification
Formula unit: smallest repeating crystal unit
Basal spacing: distance between two consecutive layer units
Structural Types (classification)
Based on Si tetrahedra / Al octahedra stacking:
1:1 (e.g. kaolinite)
2:1 (e.g. smectite, illite)
2:1:1 (e.g. chlorite)
Special forms: tubes, spheres
Different interlayer compositions (K⁺, water, cations, OH layers)
Isomorphic Substitution
Tetrahedral: Si⁴⁺ → Al³⁺
Octahedral: Al³⁺ → Mg²⁺ or Fe²⁺
Creates permanent negative layer charge
Charge Compensation
Exchangeable cations in interlayers
Positively charged Al-hydroxide layers
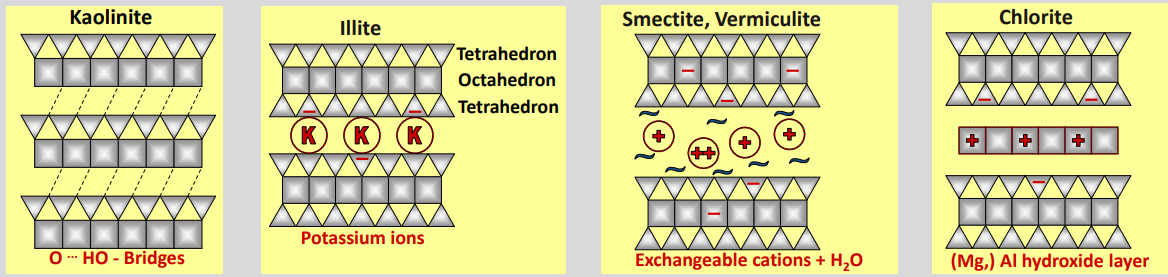
Two layer clay mineral → Kaolinite
Structure: 1 tetrahedral + 1 octahedral layer (1:1)
→ layers linked by H-bonds, no interlayerBasal spacing: ~0.72 nm
Formula: Al₂(OH)₄Si₂O₅
Origin
Forms in Si-poor, strongly weathered (tropical) environments
End product of silicate weathering
Properties
White, non-expanding, no swelling/shrinking
Used for ceramics (porcelain)
Soil relevance
No nutrients in structure
Very low CEC (little isomorphic substitution)
Typical of low-fertility soils
Everything that could leach or so has happened, really ancient, not fertile, really deep
Three layer clay minerals (Illite, Smectite, Vermiculite)
Illite (2:1)
Derived from mica
Basal spacing ~1 nm
K⁺ fixed in interlayer → almost no swelling → K keeps layers together, not exchangeable
Important K source for soils
Smectite (2:1)
Often from basic igneous rocks
Expandable (1–2 nm)
Strong swelling/shrinking
Interlayer: hydrated, exchangeable cations
High CEC, high water retention
Hydrated cations in the middle
Available for plants, really fertile
Vermiculite (2:1)
Forms from biotite/muscovite
Expandable up to ~2 nm
Strong water retention
Interlayer cations partly fixed, partly exchangeable
Four layer clay mineral (Chlorite)
Chlorite (2:1:1 clay mineral)
Primary and secondary clay mineral
Structure: 2:1 layers + hydroxide interlayer (Mg-, Al-, or Fe-hydroxides)
Not expandable
Very low to no cation exchange capacity (CEC)
Layered clay minerals
Kaolinite (1:1)
No true interlayer; layers held by H-bonds (O–H···O) → non-expandable, very low CEC → no shrink or swellIllite (2:1)
K⁺ fixed in the interlayer → strong layer bonding, no swelling, moderate CEC → hold by KSmectite / Vermiculite (2:1)
Hydrated, exchangeable cations + H₂O in the interlayer → expandable, high CEC → lot’s of exchange, swelling and shrinkingChlorite (2:1:1)
Mg/Al hydroxide interlayer → rigid structure, non-expandable, very low CEC.
Further clay minerals
Mixed layers → transitions between layered forms, more reactive than pure minerals
Examples → Imogolite and Allophane
Clay mineral transformation
Phyllosilicates (layer silicates)—especially micas—are the main precursors of clay minerals.
Physical weathering opens layer edges and removes interlayer K⁺, which is replaced by larger Ca²⁺ and Mg²⁺.
This drives the transformation sequence mica → illite → vermiculite / smectite, and with further alteration can lead to kaolinite or chlorite.
Short summary clay minerals
Important secondary new formations in the soil (see «Weathering» for details)
They are subject to constant change, but their stability is usually significantly higher than that of primary silicates
Importance for soil
Clay minerals are components of the finest fraction of the soil, < 2 µm, = clay fraction
Storage for nutrients whose leaching is prevented
Microstructure makers in soil: particles adhere to each other, bind to themselves and to other coarse particles, expand and shrink etc..
Oxide and hydroxide of Fe, Al, Mn
They are final products of weathering of silicate minerals.
Weathering releases Fe, Al and Mn ions into the soil solution.
These ions are oxidised and then precipitate.
This forms iron, aluminium and manganese oxides and hydroxides.
Aluminium hydroxides: gibbsite
An aluminium hydroxide mineral formed during strong weathering.
Colourless to white.
Built from Al-octahedra (layered structure).
Forms only when silicon is very low in soil water.
Typical of intense weathering, especially in tropical regions.
Iron oxides and hydroxides
Iron oxides & hydroxides (overview)
Form during weathering of silicates when Fe²⁺ is released and oxidized to Fe³⁺
Give soils yellow–brown–red colours
Stable in oxic (aerobic) conditions, dissolve under reducing (anaerobic) conditions
Very important for nutrient and trace-element binding
Main types
Hematite (α-Fe₂O₃)
Red colour
Forms in warm, dry, well-aerated conditions
Typical of tropical and subtropical soils
Goethite (α-FeOOH)
Yellow–brown colour
Forms at moderate temperatures
Very stable, common in temperate soils
Lepidocrocite (γ-FeOOH)
Orange colour
Forms under reducing / waterlogged conditions
Metastable, often local and temporary
Ferrihydrite (poorly crystalline Fe oxide)
Brown
Very young, poorly ordered (“rust”)
Forms during rapid oxidation, very reactive
Schwertmannite
Yellow–brown
Forms in acidic, sulphate-rich waters
Typical product of pyrite (FeS₂) weathering
Mn oxides
Manganese (Mn) oxides
Form during weathering of silicates
Mn²⁺ is oxidized to Mn⁴⁺, precipitating as Mn oxides
Main form
MnO₂
Black colour
Forms black mottles/concretions in hydromorphic soils
Immobile under aerobic conditions
Redox behaviour
Under reducing and acidic conditions:
Mn oxides dissolve
Mn²⁺ becomes mobile and colourless
Acid soils and permanently waterlogged soils → low Mn content
Soil importance
Bind trace elements under aerobic conditions
Release trace elements under anaerobic conditions
Behaviour very similar to Fe oxides
Oxides: indicators of soil formation
Goethite (FeOOH)
Forms in humid, wet climates
Indicates moderate weathering
No hematite → conditions too wet
Hematite (Fe₂O₃)
Forms in hot, tropical climates
Indicates intense weathering
Often together with goethite
Fe and Mn translocation (shifting)
Occurs in acidic, wet soils (e.g. Podzols)
Fe and Mn are mobilized under reducing conditions
Bleaching = loss of Fe/Mn
Accumulation horizons = re-precipitation under oxic or pH change
Salts
Carbonates
Main types:
Calcite (CaCO₃): white, reacts with HCl → CO₂
Dolomite (CaMg(CO₃)₂): less soluble, Mg source
Origin:
Primary minerals (e.g. limestone)
Secondary carbonates from weathering
Soil importance:
Neutralize acids (CO₂, organic acids)
Buffer soil acidification
Increase soil pH
Sulphates & Phosphates
Sulphates (from sulphuric acid):
Anhydrite: CaSO₄
Gypsum: CaSO₄·2H₂O
Phosphates (from phosphoric acid):
Apatite: main primary P mineral
Vivianite, Strengite: secondary Fe–phosphates
Soil importance:
Phosphates = key P source for soils and biosphere
Grain size
Grain size = particle size of soil (also called texture).
It describes how soil particles are distributed by size.
Why grain size matters
Controls porosity (pore size and distribution)
Regulates water and air movement
Affects transport and retention of substances (filter function)
Influences biological activity
Controls erosion risk
Important for soil structure (microstructure)
Basic soil fractions
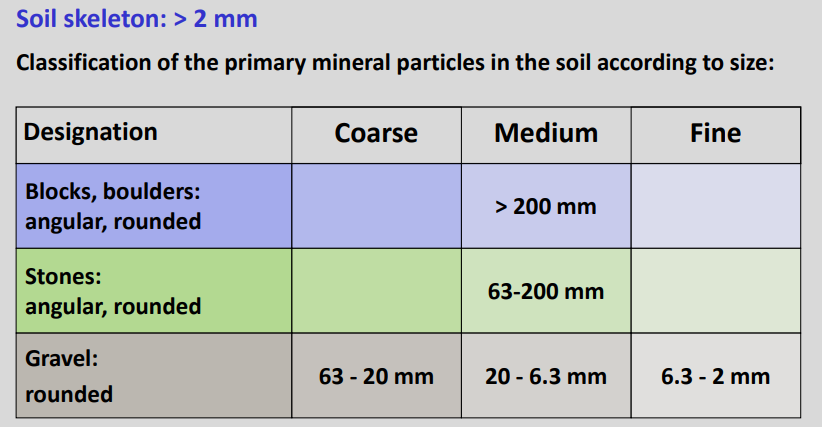
Fine earth: particles < 2 mm
Soil skeleton (coarse fraction): particles > 2 mm
Typical characteristics
Coarse fraction: mostly rounded grains, mainly SiO₂ (quartz)
Fine fraction: mostly flat/leafy particles, rich in mica and clay minerals
Soil skeleton
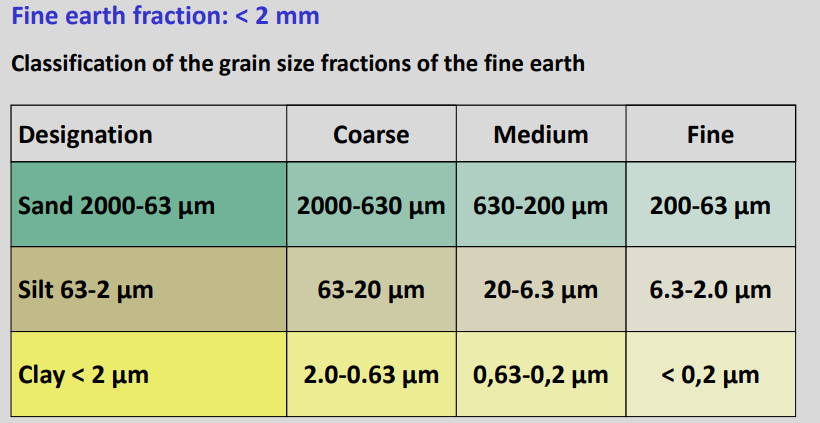
Fine earth fraction
It's used the logaritmic scale, and if you take 2 (the border between gravel and sand) then it's 63 the middle on the logaritmic scale
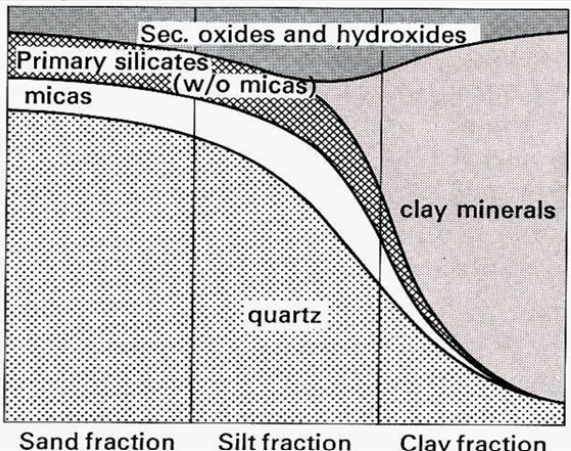
Mineral content in the grain fractions
Quartz: mainly sand (also silt)
Primary silicates (feldspars, pyroxenes, etc.): sand → silt
Micas: mainly silt (can weather to clay)
Clay minerals: clay (< 2 µm)
Fe/Al/Mn oxides & hydroxides: clay (very fine particles/coatings)
Rule of thumb:
Sand = quartz
silt = a bit of everything
clay = clay minerals and others
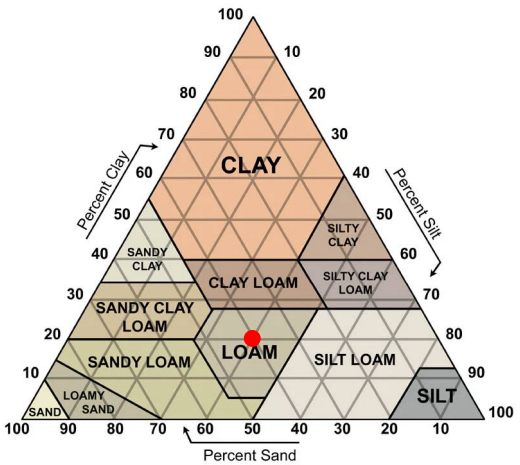
Grain size determination in the field
Finger test
Sand: gritty, loose, not sticky, no ribbon
Silt: smooth–floury, weak cohesion, no ribbon
Clay: sticky, plastic, shiny, forms long ribbon
Loam: intermediate; slightly plastic, short ribbon
Lab grain size determination
Sieve < 2 mm (fine soil only)
Remove binding material (salts & organic matter)
Measure with laser diffraction
Smaller particles → stronger laser scattering
Grain size triangle
Grain size and soil functions
Physical effects
Sand: drains fast, low water storage
Silt: best water available to plants
Clay: slow drainage, high water storage (often stagnant)
Chemical effects
Sand: few nutrients, low buffering
Clay: many nutrients, high buffering
Pollutants
Finer grains (silt–clay) retain pollutants more than sand