WJEC A Level Chemistry Unit 1
1/187
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
188 Terms
oxidation number of an uncombined element
0
oxidation number of group 1 metals
+1
oxidation number of group 2 metals
+2
oxidation number of oxygen
-2 (EXCEPT: +2 with fluorine and -1 in peroxides)
oxidation number of hydrogen
+1 (EXCEPT: -1 in metal hydrides)
what do ionic equations show
only the particles that react and the products they form, ions that do not change are LEFT OUT
atomic number
number of protons
mass number
sum of protons and neutrons
isotope
same atomic number different mass number - same protons different neutrons
radioactive decay
an unstable atomic nuclei loses energy by radiation or particle to become stable
what are alpha particles
positively charged helium nuclei
what is the charge of alpha particles
+2 charge
what is the relative mass of alpha particles
4
what can stop alpha particles
paper/Skin
what is the ionising power of alpha particles
high
what is the penetrating power of alpha particles
low
how do alpha particles behave in an electric field
deflect towards negative electric field - heavy, slow moving
how do alpha particles behave in a magnetic field
low deflection - attracted slightly
what are beta particles
high speed negatively charged electrons
what is the charge of beta particles
-1
what is the relative mass of beta particles
0
what can stop beta particles
Thin metals (aluminium)
what is the ionising power of beta particles
medium
what is the penetrating power of beta particles
medium
how does a beta particle behave in an electric field
deflect towards positive - light and fast
how does a beta particle behave in a magnetic field
high deflection - considerable deviation
what is gamma radiation
very high energy electromagnetic radiation
what is the charge of gamma radiation
none
what is the relative mass of gamma radiation
negligible
what can stop gamma radiation
lead
what is the ionising power of gamma radiation
low
what is the penetrating power of gamma radiation
high
how does gamma radiation behave in an electric field
undeflected
how does gamma radiation behave in a magnetic field
no deflection
alpha decay
mass number -4
atomic number -2
beta decay
atomic number +1
electron capture
mass number -1
positron emission
mass number -1
half life
the time taken atoms/mass in a radioactive substance to half
what is half life NOT affected by
catalysts and changes in temperature
what effect does radiation have on DNA in living cells
it damages DNA, which can cause mutations, and cell death at higher doses
what can mutations in cells lead to
formation of cancerous cells
what can high levels of radiation exposure cause
radiation burns and death
beneficial uses of radioactivity
-cancer treatment (radiotherapy)
-calculating age of plant and animal remains
-estimating geological age of rocks
-production of electricity
what is the shape of an S orbital and how many electrons can it hold
spherical and 2
what is the shape of a P orbital and how many electrons can it hold
dumbbell and 6
what is ionisation energy
the energy required to remove an electron from an atom
why do successive ionisation energies increase
greater effective nuclear charge
-as each electron is removed each shell is drawn slightly closer to the nucleus, therefore increased nuclear attraction needing more energy to break.
equation for frequency
c = f λ
(c = speed of light)
(f = frequency)
(λ = wavelength)
what happens if atoms are given energy (via heating or electrical field)
electrons get excited and are promoted from a lower energy level to a higher energy level - when the source of energy is removed the electrons fall from their higher energy level and from their excited state to a lower energy level and energy is released as a photon (quantum of energy)
equation for energy
e = h f
(e = energy)
(h = plank's constant)
(f = frequency)
FREQUENCY IS PROPORTIONAL TO ENERGY AND WAVELENGTH IS INVERSELY PROPORTIONAL TO ENERGY
balmer series
visible region, electrons return to n = 2
lyman series
ultraviolet, electrons return to n = 1
relative atomic mass
average mass of one atom of the element relative to 1/12 the mass of a carbon-12 atom
relative isotopic mass
mass of one atom of an isotope relative to 1/12 the mass of a carbon-12 atom
relative formula mass (M r)
the total average mass of all the atoms in the formula relative to 1/12 the mass of a carbon-12 atom
what can be used to find the relative atomic mass of an element
a mass spectrometer
what does a mass spectrometer measure
the relative mass of each different isotope of an element
the relative abundance of each isotope of the element
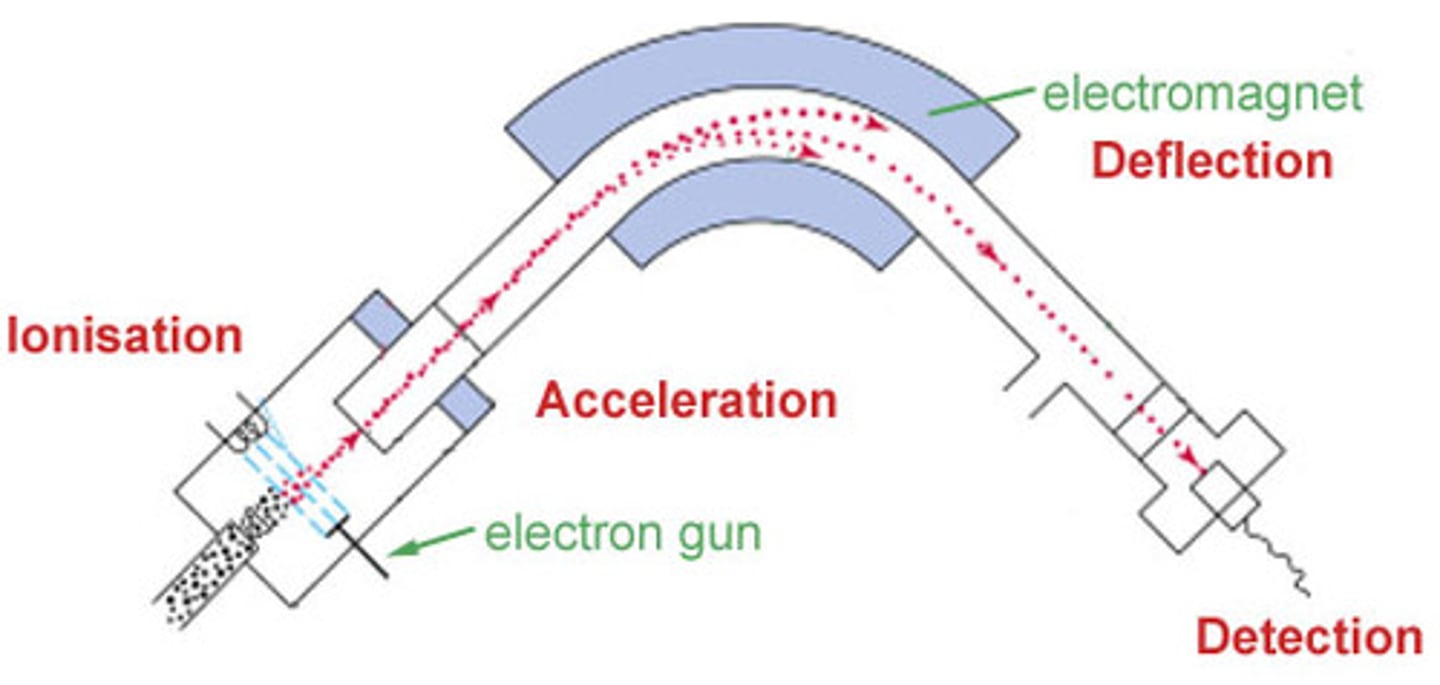
what does a mass spectrometer look like
why is a vacuum used inside
it's important that air molecules do not interfere with the movement of the ions
stages of mass spectrometry
vaporisation, ionisation, acceleration, deflection, detection
ionisation in a mass spectrometer
vapourised sample passes into the ionisation chamber and the sample particles are bombarded with a stream of electrons, some of the collisions knocking an electron out of the particles to make positive ions
acceleration in a mass spectrometer
the positive ions are accelerated to a high speed by an electric field
deflection in a mass spectrometer
ions are deflected by magnetic field, the lighter ones being deflected more than the heavier ones and ions with 2 positive charges are deflected more than those with 1 - THESE 2 FACTORS ARE COMBINED IN THE M/Z RATIO (MASS/CHARGE)
detection in a mass spectrometer
only ions with a given M/Z ratio make it to the ion detector - electrons are transferred from detector plate to positive ion which creates a current
the larger the current, the higher abundance of the isotope
determining relative atomic mass of an element (A r)
(if on a graph with the lines, X axis x Y axis over 100 for A r
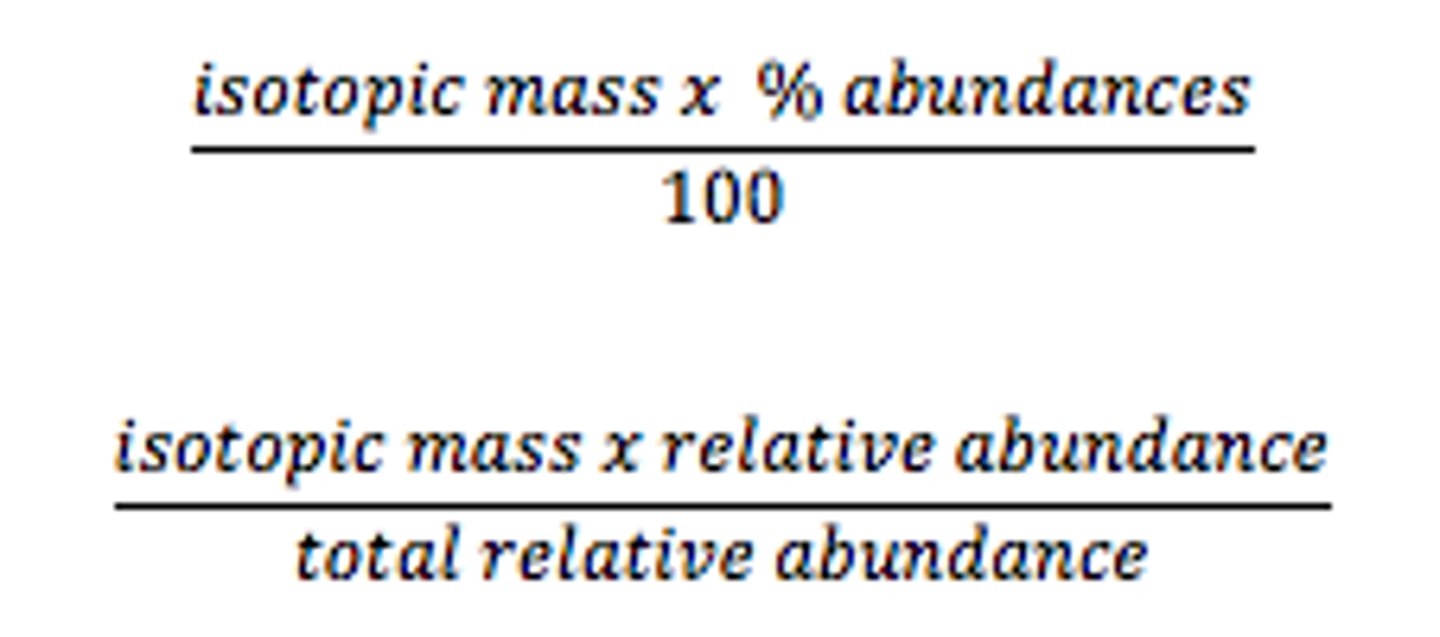
what is a mole
the amount of a substance in grams that contains as many particles as there are atoms in 12 g of carbon-12
1 mol = 6.02x10'23 (avogadros constant!)
what is molar mass
the mass of one mole of a substance
moles equation
moles = mass/Mr
atom economy equation
percentage yield = (mass of product obtained ÷ maximum theoretical mass) X 100
ionic bonding
when a metal and non-metal react together, the metal atom loses electrons and becomes a cation (+) and the non-metal gains electrons and becomes an anion (-)
strong electrostatic forces of attraction between these
dot cross diagrams (steals electrons)
covalent bonding
when non metals bond together, each atom gives one electron to form a bond pair in which the electron spins are opposed - the bond is the electrostatic attraction between positive nuclei of bonded atoms and shared electrons between them
dot cross diagrams (electrons shared, overlaps)
coordinate bonding
a covalent bond where both electrons come from one atom
what is electronegativity
a measure of how strongly atoms attract electrons in a covalent bond
-the higher the electronegativity value, the better the element can attract bonding electrons
-Strongest at top right of Periodic table (Li)
polar bonds
one atom slightly negative, one atom slightly positive
bonding between molecules
intermolecular
bonding within molecules
intramolecular
intermolecular bonding
weak, governs physical properties (eg. boiling temp)
intramolecular bonding
strong, governs chemical reactivity
dipole
a molecule that contains both positively and negatively equal charges
Atom
The smallest part of an element that can exist. All substances are made up of atoms.
Compound
A substance that combines two or more different elements through the formation of chemical bonds.
Ion
Formed when an atom/molecule loses or gains electrons. This gives it an overall charge - a positive charge if it has lost at least one electron and a negative charge if it has gained at least one electron.
Ionic equation
A chemical equation that involves dissociated ions.
Molecular formula
The actual number of atoms of each element in a molecule.
Oxidation number
The charge of an ion or a theoretical charge of an atom in a covalently bonded compound assuming the bond becomes ionic.
State symbol
Symbols which show the physical state of the substance during the reaction, they are usually in brackets: gas (g), liquid(l), solid(s) and aqueous(aq). Aqueous means the substance is dissolved in water.
Absorption spectra
A spectrum of frequencies of electromagnetic radiation that has been transmitted through an atom or molecule, that shows dark bands due to the absorption of the radiation at those specific wavelengths.
Alpha-decay
A type of radioactive decay, during which an atomic nucleus loses two protons and two neutrons. An alpha particle is equivalent to a helium nucleus. It reduces the atomic number by two and the mass number by four, making the element more stable.
Atomic number
Total number of all the protons in the nucleus. It increases as you go across the periodic table.
Beta-decay (positron emission)
A type of radioactive decay, during which a beta particle is lost, which is equivalent to an electron and a neutron turns into a proton or a proton turns into a neutron. This changes the atomic number by one, but the mass number remains the same.
d orbitals
Regions in which up to two electrons can be found. There are five d orbitals, so in total, the d subshell can hold up to 10 electrons.
Electromagnetic spectrum
The range of frequencies of electromagnetic radiation and the respective wavelengths.
Electron capture
Occurs to make an atom more stable, an electron from the atom's inner shell of electrons moves into the nucleus where it joins with a proton, this forms a neutron and a neutrino. The neutrino formed is expelled from the atom's nucleus.
Electron configuration
The arrangement of electrons into orbitals and energy levels around the nucleus of an atom/ion. E.g. Ca: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s².
Electron transition
When an electron absorbs energy and moves from a low energy orbital to a vacant higher energy orbital.
Emission spectra
A spectrum of frequencies of electromagnetic radiation that has been emitted by an atom or molecule undergoing a transition from a state with higher energy to a state with lower energy.
Energy level
The shell that an electron is in.
First ionisation energy
The energy required to remove 1 mole of electrons from 1 mole of gaseous atoms to form 1 mole of gaseous 1⁺ ions. For example, Mg(g) → Mg⁺(g) + e⁻.
Frequency
The number of wave oscillations per second.