Nuclear Physics
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
What is nucleon number?
The sum of the number of protons and neutrons in the nucleus
What is the atomic number?
The total number of protons in the nucleus.
Outline Rutherford's alpha scattering experiment.
High speed alpha particles were fired at a very thin sheet of gold foil. The deflections of the particles were measured and conclusions were drawn.
What was observed in the Alpha scattering experiment?
- Most of the alpha particles passed straight through the gold atoms
- Some of them were deflected
- A few of them were deflected backwards
What is thermionic emission?
Thermionic emission is the release of electrons due to heating.
Explain why electrons are released from a heated filament.
As the filament heats up, free electrons inside the metal gain kinetic energy. When the surface electrons gain sufficient energy, they are released from the surface.
What will happen to a beam of electrons if it is passed through a potential difference?
The beam of electrons will be accelerated since work is done by the potential difference.
How do you calculate the energy transferred to an electron, when it is accelerated across a potential difference
Energy = Charge x Potential Difference
Energy = eV
What happens when a beam of electrons is directed into a magnetic field?
The electron beam will be deflected, since magnetic fields apply forces on moving charges.
What is the magnitude of the force experienced by a moving electron in a magnetic field?
Force = Magnetic Flux Density x Charge x Velocity
F = Bev
If the electrons are moving perpendicular to the field lines, which direction will the magnetic force act?
The force will act perpendicular to both the electron and field directions.
Describe the shape of the path of a beam of electrons passing through magnetic field.
The beam will produce a circular path since the magnetic force always acts perpendicular to the electrons' motion. This means it acts as a centripetal force and produces a circular path.
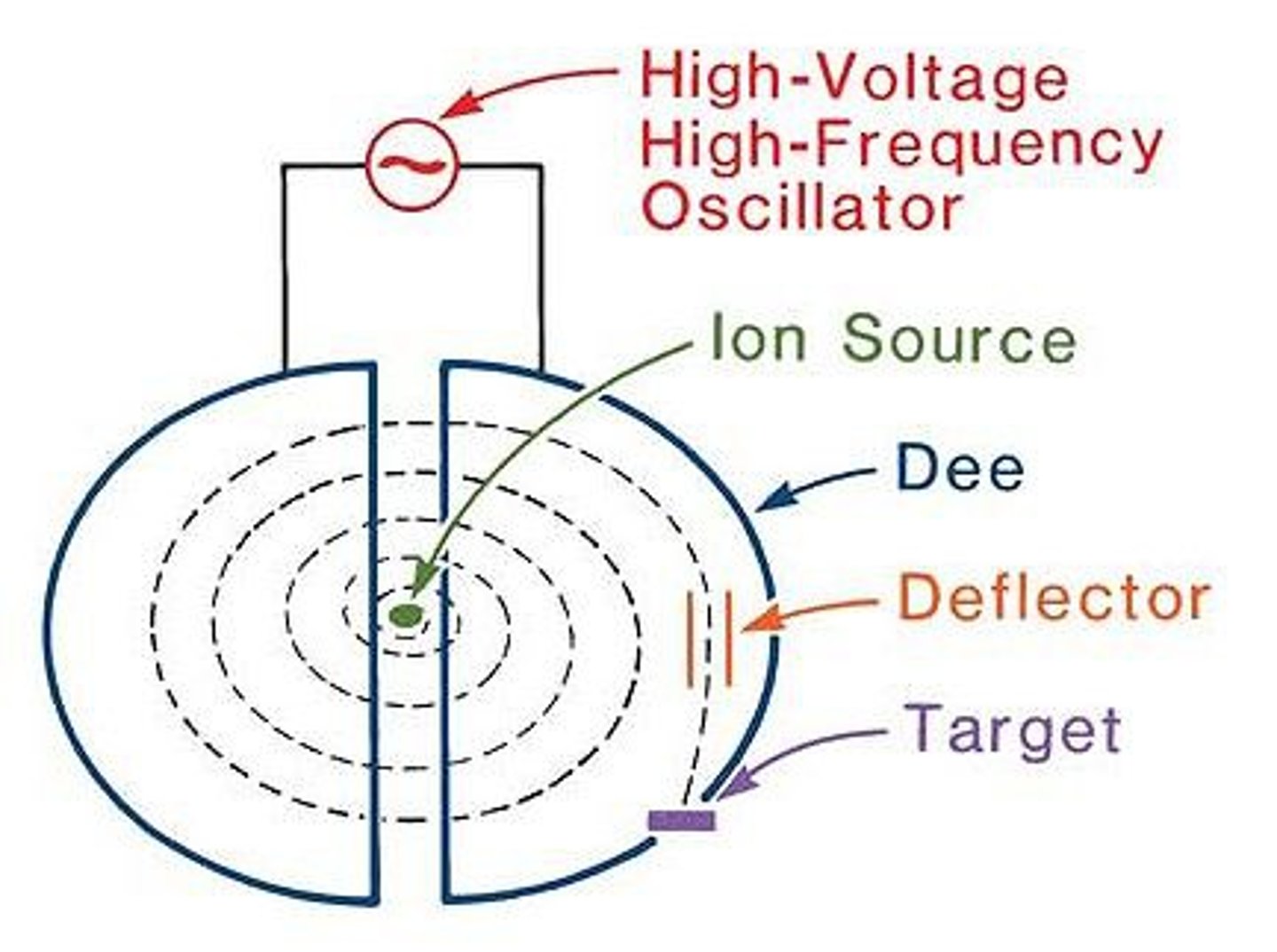
What is a cyclotron?
A cyclotron is a particle accelerator that uses magnetic fields to accelerate particles in circular paths. This allows higher speeds to be reached, without the limitation of the accelerator's length
Describe the basic composition of a cyclotron.
Cyclotrons consist of two D-shaped paths which are separated by a small gap. An alternating potential difference is applied across the gap
How does a cyclotron work?
An electron beam is passed into the cyclotron, where it is deflected into a circular path by a perpendicular magnetic field.
When the beam reaches the gap, it is accelerated by a potential difference. This increases the speed of the beam, causing the radius of the path to increase. This process repeats every half circle.
State the equation used to calculate the circular radius of an electron beam deflected in a magnetic field.
Radius = mv/BQ
What two equations must you combine to derive the radius equation?
1. Centripetal Force = mv²/r
2. Magnetic Force = BQ/v
Centripetal Force = Magnetic Force
State the mass-energy equation.
E = mc²
What is 1 MeV in Joules?
(1.6x10¹⁹) x 10⁶ = 1.6 x10⁻¹³J
In the quark-lepton model, what are the four main categories of particles?
1. Baryons
2. Mesons
3. Leptons
4. Photons
Describe the quark composition of a baryon
Baryons are made up of three quarks
Describe the quark composition of a meson
Mesons are made up of a quark and antiquark pair
What category of particles are classed as fundamental particles?
Leptons
Give two examples of leptons.
1. Electrons
2. Neutrinos
What category of particles do pions belong in?
Mesons.
Give two examples of baryons.
1. Protons
2. Neutrons
What did the symmetry of the quark-lepton model predict the existence of?
The top quark.
What is an antiparticle?
An antiparticle is one that has the same mass but opposite charge and conservation numbers to its corresponding particle.
What is the antiparticle of a proton?
An antiproton.
What is the antiparticle of an electron?
A positron.
Name four things that are always conserved in a particle interaction.
1. Mass/Energy
2. Baryon Number
3. Lepton Number
4. Charge
Describe the conservation of lepton number.
The lepton number for each specific type of lepton must be the same before and after an interaction.
Changes in Atomic Models
- In the early days (1803) Dalton thought that everything was made up of indivisible particles.
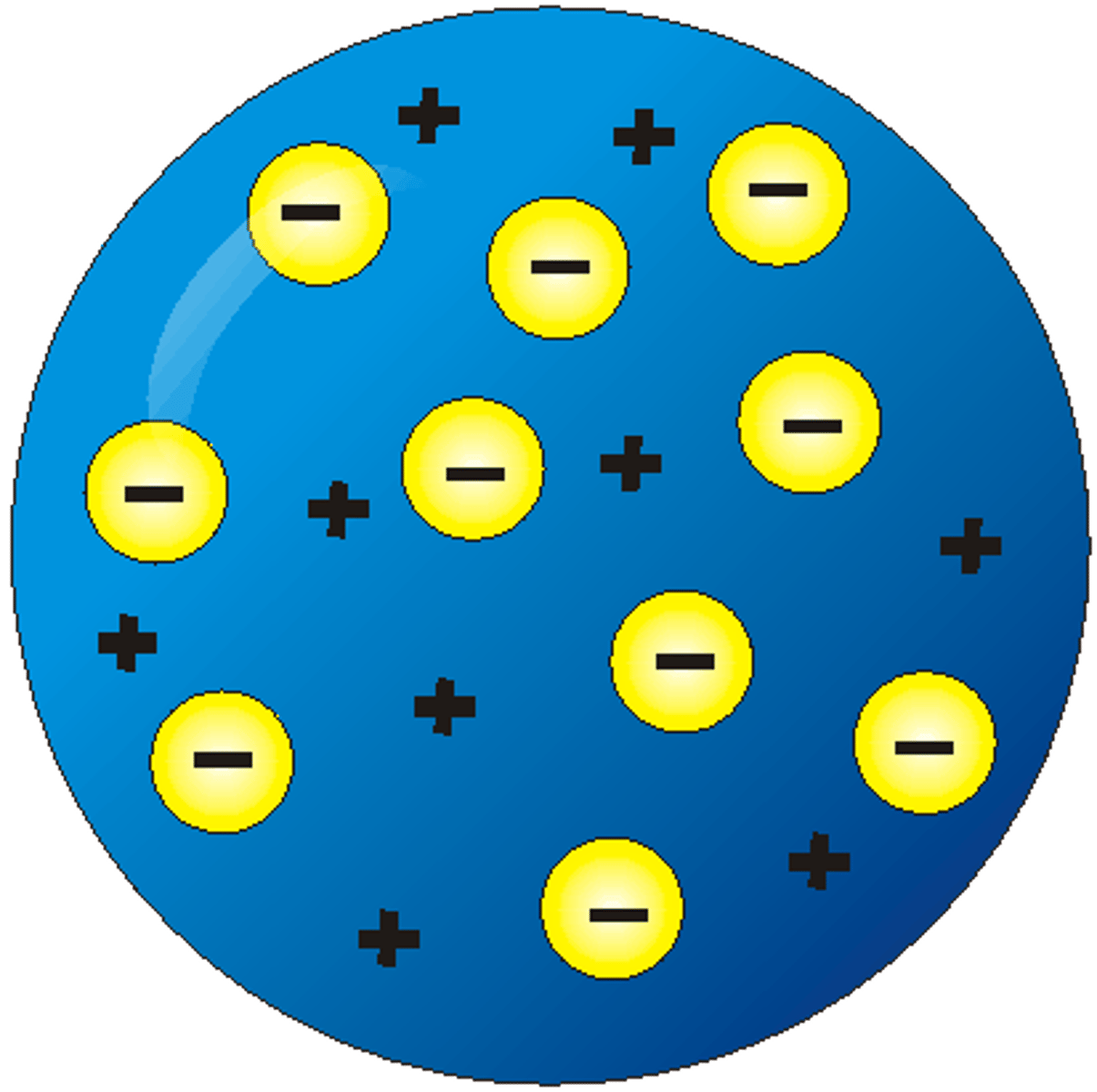
- It was then though later in 1897 that atoms were positive bodies of matter with negative electrons dispersed throughout them. This was named the plum pudding model by Thompson.
- Later, it was discovered in 1911 by Rutherford that atoms were mostly empty space, had a small dense nucleus and had electrons orbiting them.
- Then in 1913, it was discovered these electrons orbit in shells and emit EM radiation when they move between them.
- Then in 1913 it was discovered these electrons orbit shells and emit EM radiation when they move between them.
- 1919, the proton was discovered, followed by the neutron
- Now we believe that electrons exist in subshells and that the nucleons are quarks - this is referred to as quantum mechanics
Plum Pudding Model
Thomson's Plum Pudding Model was the accepted model of the atom prior to experiment such as Rutherford's Alpha Scattering experiment, which disproved. The model described the atom to be a ball of positive charge, with negatively charged electrons evenly distributed throughout it.
Rutherford Scattering Experiment:
This experiment provided evidence to support the currently accepted Bohr model of the atom, and discounted the the believed Plum Pudding model.
Alpha particles were fired at a thin sheet of gold foil. If the plum pudding model was true, the particles were expected to pass through with only sight deflections.
However, in reality:
- Most passed through with no deflection, suggesting that the atom is mostly empty space and that the nucleus is very small.
- Some were deflected by large angles, suggesting that there is a positively charged nucleus that repels the positive alpha particles.
- A few were deflected by more than 90 degrees, suggesting that the central nucleus was very small, but also very dense since it changed the direction of the very fast moving alpha particles
Investigating the nucleus:
- Nucleon number: The number of neutrons and protons in the nucleus
- Proton number: The number of protons in the nucleus
High energies are required to investigate the structure of nucleons due to their small size. This is because the higher the energy of matter (such as electrons), the smaller their De Broglie wavelength, and so they are more suitable for investigating smaller objects (as the resolution increases).
Fundamental particles are particles that cannot be broken down into any smaller constituent parts. Most types of matter however are not fundamental and can be broken down into quarks.
Quarks can only ever be found in threes or in an quark and antiquark pair. Quarks can never exist alone, due to a principle known as quark confinement.
Quarks come in three main types:
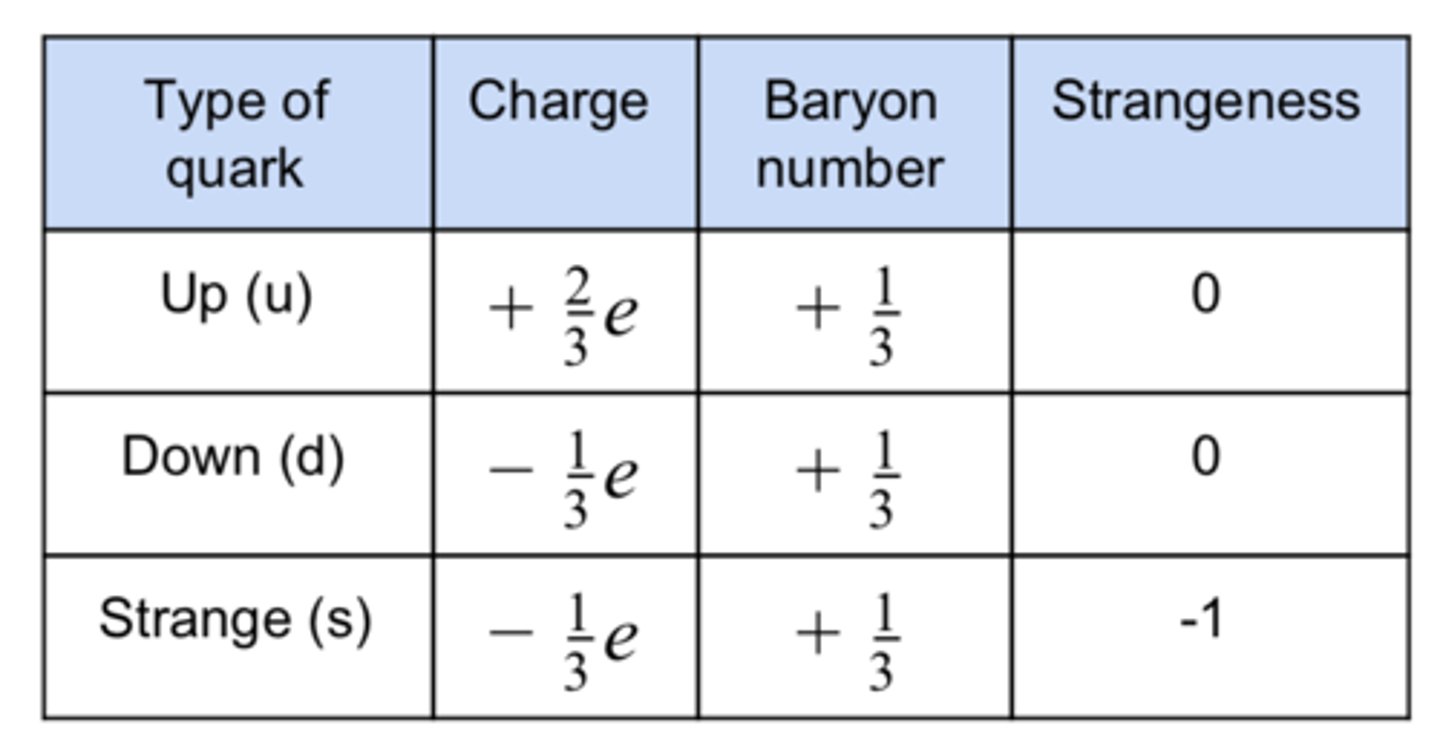
Quark-Lepton Particle Model
The quark-lepton model splits particles into four main categories:
1. Baryons consist of three quarks and both protons and neutrons are types of baryons. You should know that the only stable baryon is the proton, so all other baryons eventually decay into the proton.
2. Mesons consist of a quark and antiquark pair and include pions which are the exchange particle for the strong nuclear force. Kaons are a heavier form of meson, which decay into pions.
3. Leptons are fundamental particles and include the electron. The only interact in the weak nuclear force.
4. Photons
Another term that you should be aware of is hadrons. Hadrons are the wider particle group that contains both baryons and mesons. All hadrons interact through the strong nuclear force and are made up of quarks
Exchange particles are the force carriers for the force carriers for the fundamental forces. They transfer energy, momentum and sometimes charge. The size of the exchange particle determines the range of the force; the bigger the particle, the shorter the range.
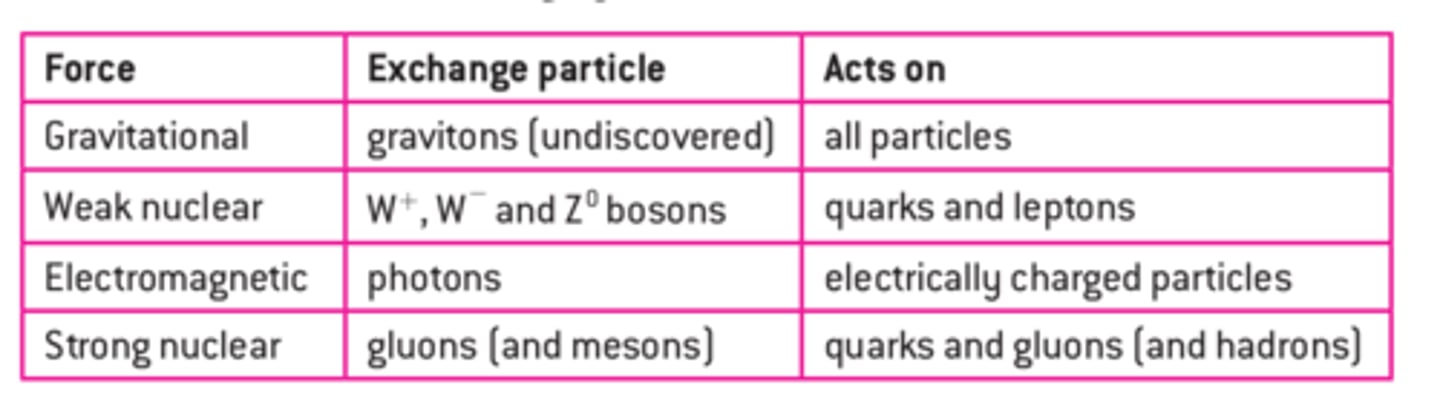
Feynman diagrams show particle interactions. Time moves from the bottom to the top and their position is shown by their position horizontally.
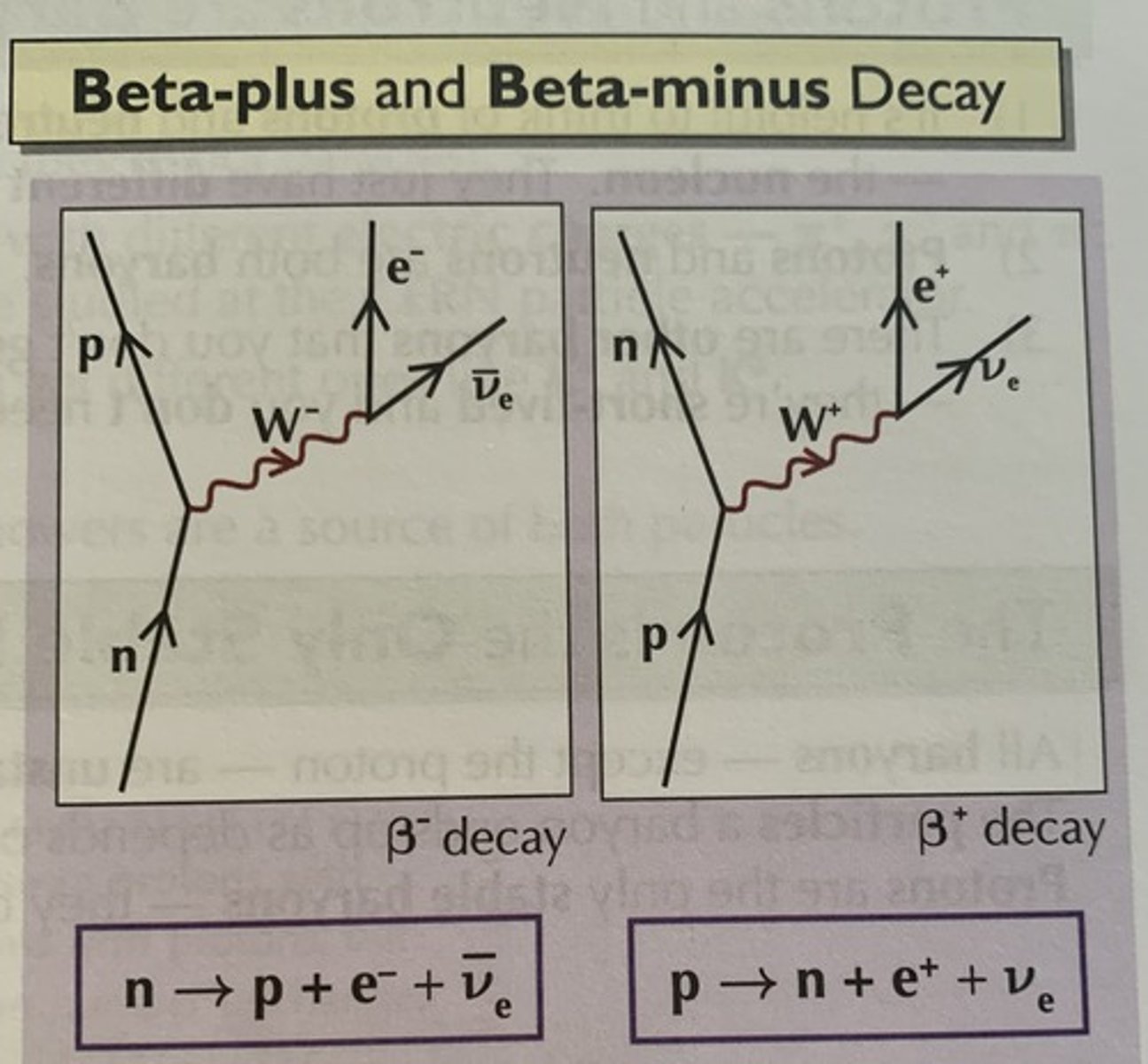
The following properties are always conserved:
Charge: Always indicated by the particle.
Baryon number: 0 except for baryons which are +1
Lμ: 0 except for a muon and muon neutrino which are +1
Lₑ: 0 except for an electron and electron neutrino which are +1
Strangeness: K⁺ and Kᴼ are +1, K⁻ and anti-Kᴼ are -1
If it is an antiparticle all of these are opposite, +1 becomes -1 and 0 remains 0.
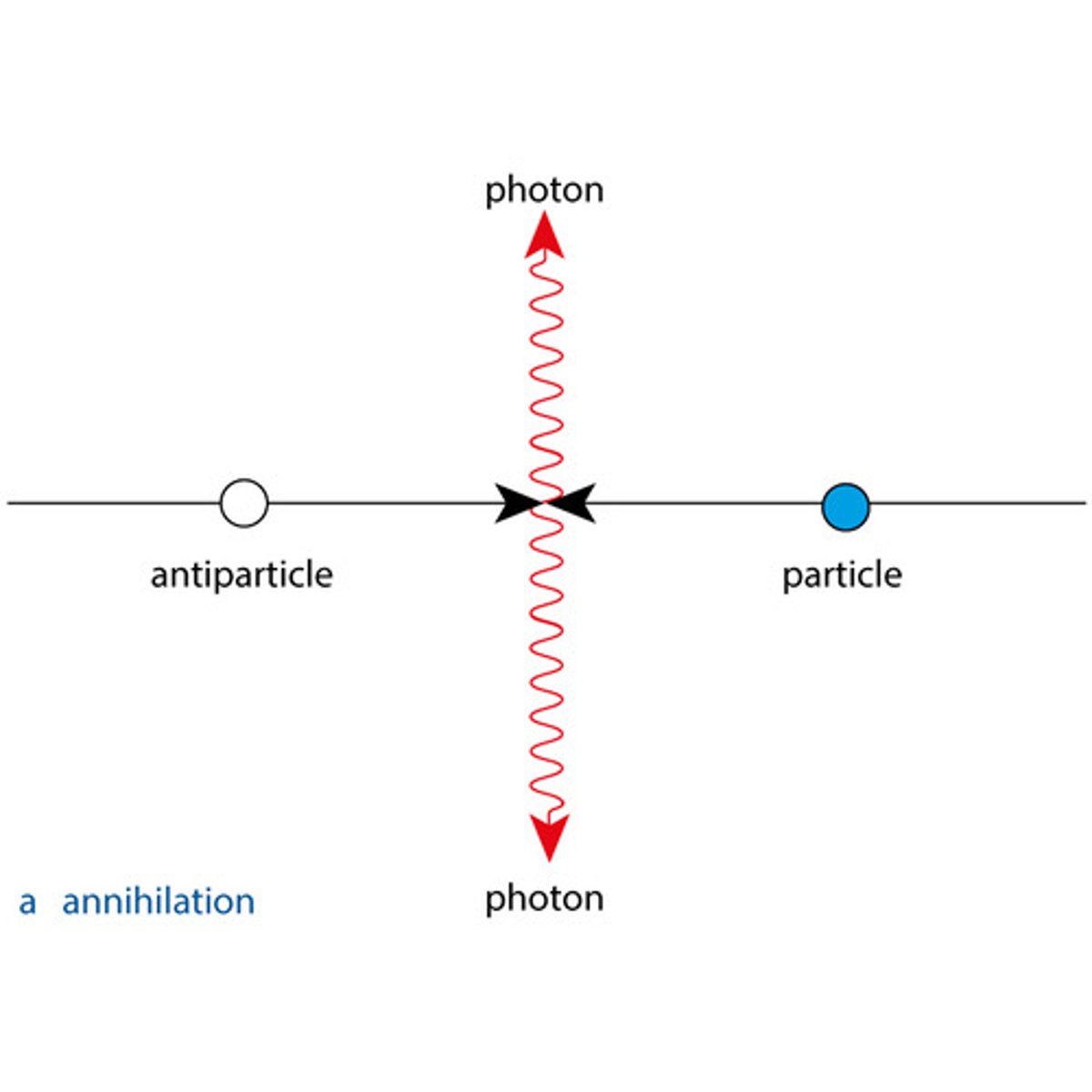
Every particle has an antiparticle. Antiparticles have the same mass/rest energy but opposite charges and opposite quantum numbers compared to their normal matter counterparts.
A particle and corresponding antiparticle annihilate each bother in an annihilation reaction, releasing two photons. The photons must go in opposite directions to conserve momentum.
2Eₘᵢₙ = 2Eᵣₑₛₜ
Eₘᵢₙ = Eᵣₑₛₜ
hf = Eᵣₑₛₜ + Eₖ
The mass-energy equivalence is the concept that mass and energy are interchangeable. This can be expressed as the following equation:
E = mc²
You can use the above equation to calculate the energy transferred when a particle and antiparticle are produced or annihilated, by substituting their collective mass with the m in the equation.
An easy method of producing an electron beam is through thermionic emission. This is when a metal is heated, resulting in free electrons inside the metal gaining sufficient kinetic energy to leave the metal's surface. The most common method of achieving this is with a wire filament:
- A current is passed through the filament
- The filament heats up, and free electrons gain kinetic energy
- Electrons that gain kinetic energy are released from the surface
To accelerate the electrons that are released, and to produce a beam, an anode is placed opposite the filament. There is a vacuum between the filament and the anode, which allows the electrons to be accelerated across the gap, due to the electric field between them. There is a small hole in the anode that the electrons then pass through.
If you know the accelerating voltage, it is possible to calculate the speed of the electrons as they pass through the hole. All the kinetic energy that electrons have, comes from the work done by the potential difference, and so: eV = ½mv²
Deflection in a Magnetic Field:
In a magnetic field, moving charged particles will experience a magnetic force perpendicular to their motion. This magnetic force act as a centripetal force and so the particles will follow a circular path. The radius of their path can be calculated by using the equation derived:
The magnetic force on the particles is the centripetal force causing their circular motion and so:
BQv = mv²/r
r = mv/BQ
r = p/BQ
An application of the circular deflection of charged particles in a magnetic field is a type of particle accelerator called a cyclotron.
A cyclotron is formed of two semi-circular electrodes, with a uniform magnetic field acting perpendicular to the plane of the electrodes, and an alternating voltage applied between the electrodes. The charged particles move from the center of one of the electrodes, and are deflected in a circular path by the magnetic field.
Once the particle reach the edge of the electrode they begin to move across the gap between the electrodes, where they are accelerated by the electric field, meaning the radius of their circular path will increase as they move through the second electrode. This process repeats several times until the required speed is reached by the particles and they exit the cyclotron.