Intro to organic chemistry
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
Empirical formula
Simplified whole number ratio of atoms
E.g. C5H12
Molecular formula
The actual number of atoms of each element in the molecule
E.g. C5H12
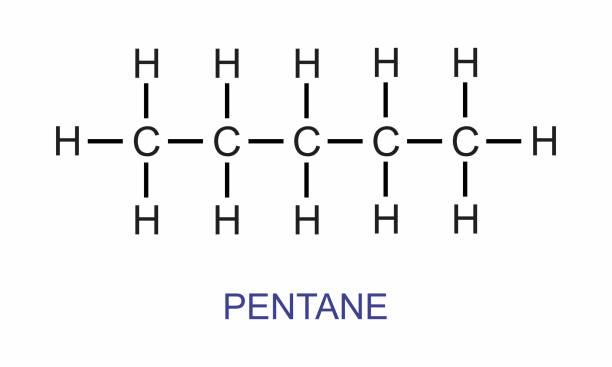
Displayed formula
Shows every atom and every bond in the molecule
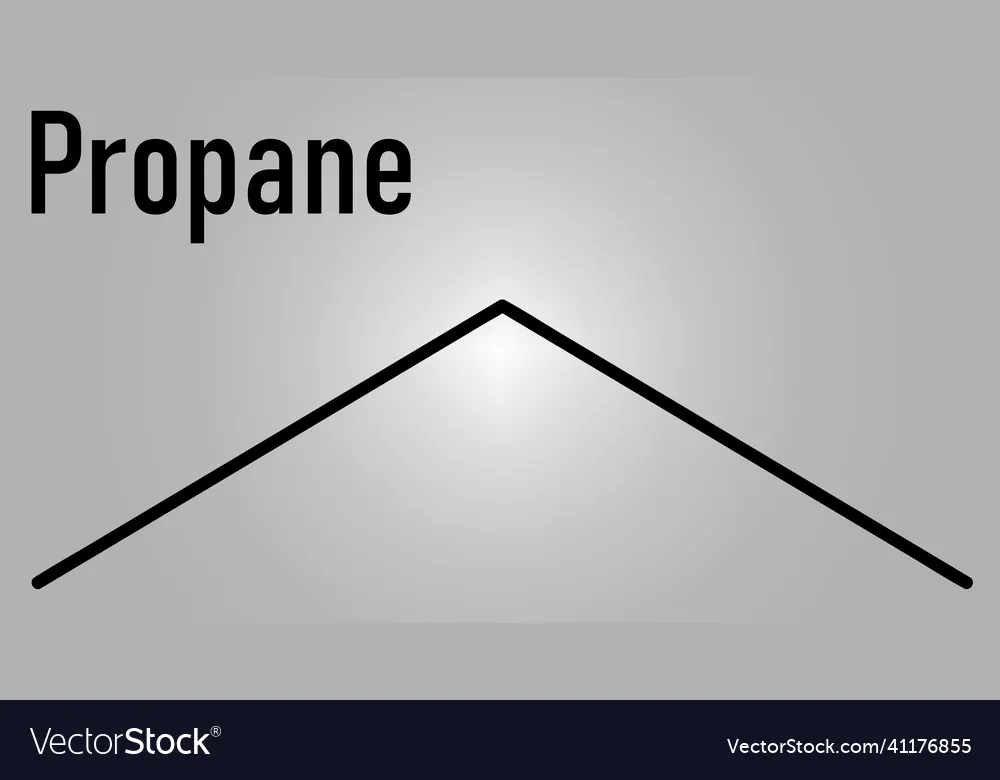
Skeletal formula
All of the carbon and hydrogen atoms are removed from carbon chains leaving just a carbon skeleton with functional groups attached to it
Structural formula
Shows the arrangement of atoms in a molecule in a simplified form
No bonds shown
Each carbon is written separately
E.g. Pentane - CH3CH2CH2CH2CH3
Homologous series
A family of organic compounds with the same functional group but different chain lengths
What are the key characteristics of a homologous series ?
Same general formula
Similar chemical activity
Melting and boiling points increase as carbon chain length increases due to increased intermolecular forces
What is the difference between an Alkane and an Alkene ?
Alkenes have a C=C double bond but Alkanes only have a C-C single bond
What is the functional group of an Alkene ?
C=C
What is the functional group of a Halogenoalkane ?
— X (e.g. - F, - Br, - Cl, - I )
X is always a halogen (group 7 element)
What is the functional group of an Alcohol ?
— O — H
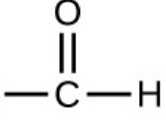
What is the functional group of an Aldehyde ?
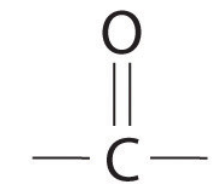
What is the functional group of a Ketone ?
No hydrogen atom
C=O is between 2 carbons in a chain
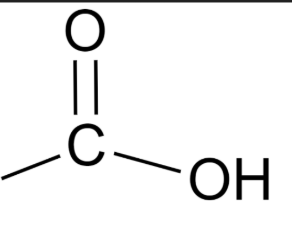
What is the functional group of a Carboxylic acid ?
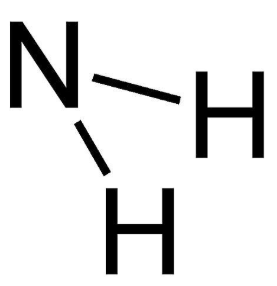
What is the functional group of a Primary Amine ?
What is the functional group of a Nitrile ?
