aromatic hydrocarbons I: structures and reactions
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
aromatic compounds
benzene is a resonance hybrid of these resonance structures. Individual resonance forms are imaginary, not real. The real structure is a composite, or resonance hybrid, of the different forms
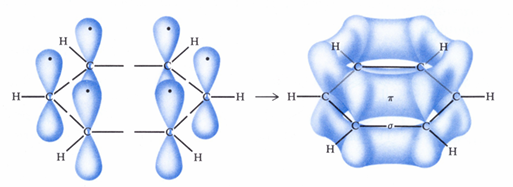
orbital representation
each carbon of benzene has a 2pz orbital with its one electron sideways overlaps the 2pz orbitals of its two neighbors to give an extended pie cloud containing 6 pie electrons
all carbon-carbon bonds are equal length
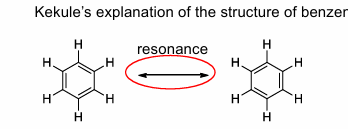
discovery of benzene
1852 Michael faraday
kekule
proposed structure of benzene
kekule’s structure of benzene
the correct explanation is that it is not two structures in equilibrium but a single structure which is a resonance between the two above
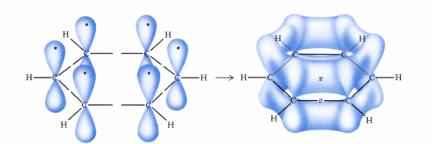
Huckel’s rule for aromaticity
to be aromatic, a compound should have a planar ring(s), each atom in the ring has a pz orbital and there must be (4n + 2)pie electrons (n = integer). i.e. for benzene n = 1
benzene has an aromatic sextet or an aromatic pie cloud containing 6 pie electrons
benzenoid aromatic compounds
benzene
toluene
1,2-dimethylbenzene
aniline
phenol (aromatic alcohol)
hexamethylbenzene
naming Di-substituted benzenes
the prefixes ortho-, meta, and para are commonly used for 1,20, 1,3- and 1,4- positions respectively
polycyclic and heterocyclic aromatic compounds
Compounds that contain multiple fused aromatic rings or have one or more heteroatoms in the ring structure. These include naphthalene (10 pie electrons (n=2)) and anthracene (14 pie electrons (n=3))
electrophilic substitution
typical reactions are substitution reactions since these preserve the aromatic sextet (6 pie electrons)
typically electrophilic substitution since in these reactions electrophiles react with electron rich aromatic ring
F2 reacts violently without catalyst
Br2 reacts in the presence of catalyst AlBr3
no light is needed to initiate the reaction (not free radical mechanism)
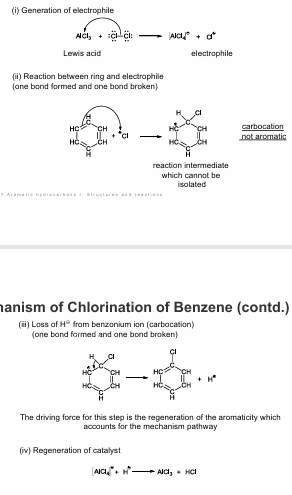
mechanism of chlorination of benzene
i) generation of electrophile
ii) reaction between ring and electrophile (one bond formed and one bond broken)
ii) loss H+ from Benz onium ion (carbocation) (one bond formed and one bond broken)
the driving force for this step is the regeneration of the aromaticity which accounts for the mechanism pathway
iv) regeneration of catalyst
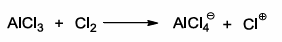
AlCl3 as a Lewis acid
AlCl3 acts as a Lewis acid (electron pair receptor) abstracting Cl- and Cl2 thereby generating the key electrophile Cl+
Cl- acts as a Lewis base (electron pair donor)
BF3 and all group III trihalides (e.g. AlCl3) are Lewis acids since there have only six electrons around central atom
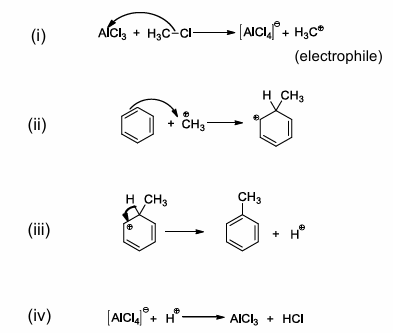
Friedel Craft’s alkylation of benzene
important carbon-carbon forming reaction
reaction does not work with aryl halides such as chlorobenzene
Friedel crafts alkylation of benzene mechanism
follows the same general reaction pathways as chlorination
nitration of benzene
i) formation of the electrophile
the rest follows the same general reaction pathways as chlorination and alkylation