Chemistry 3.3- Halogenoalkanes
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
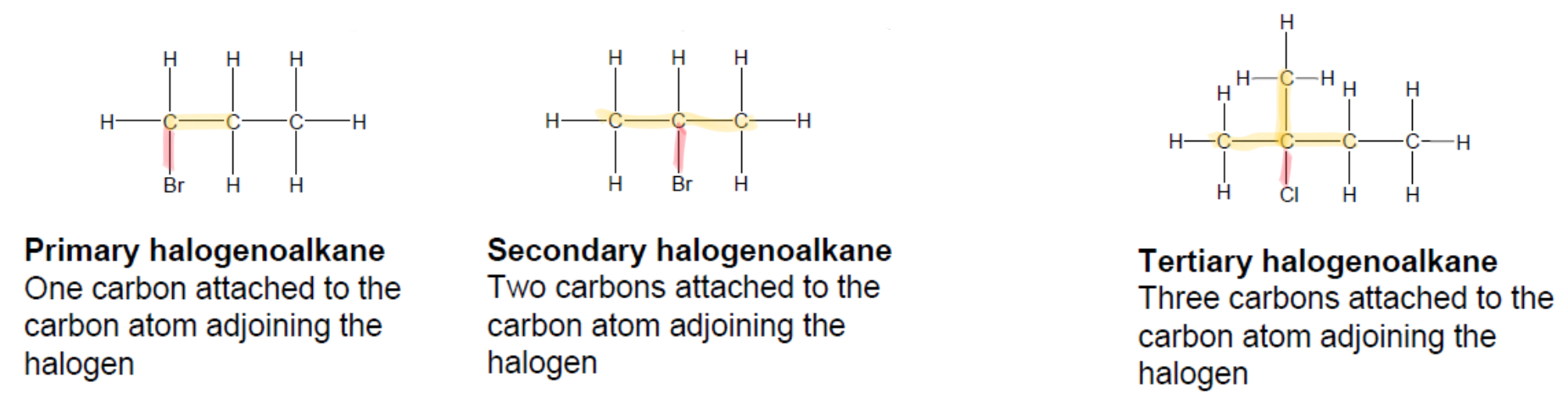
What are primary, secondary and tertiary haloalkanes?
In a primary haloalkane, the halogen is bonded to a carbon which is bonded to one other carbon
In a secondary haloalkane, the halogen is bonded to a carbon which is bonded to two other carbons
In a tertiary haloalkane, the halogen is bonded to a carbon which is bonded to three other carbons
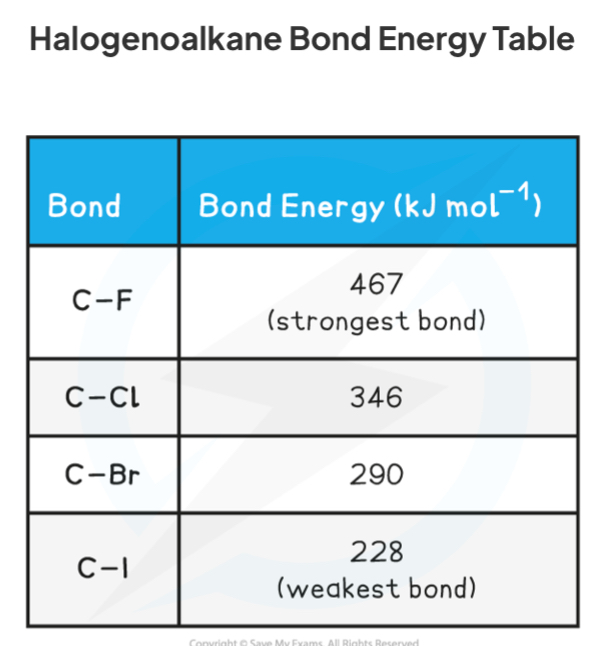
Why do haloalkanes have different rates of reaction?
The large difference in electronegativity between the carbon atom and the halogen atom in a haloalkane results in a polar bond
This means they can undergo nucleophilic substitution reactions, where the carbon-halogen bond is broken
The halogens have different electronegativity, so the carbon-halogen bonds vary in strength, having different bond enthalpy
Lower bond enthalpy means a more reactive haloalkane, and higher rate of reaction, as the bond is broken more easily
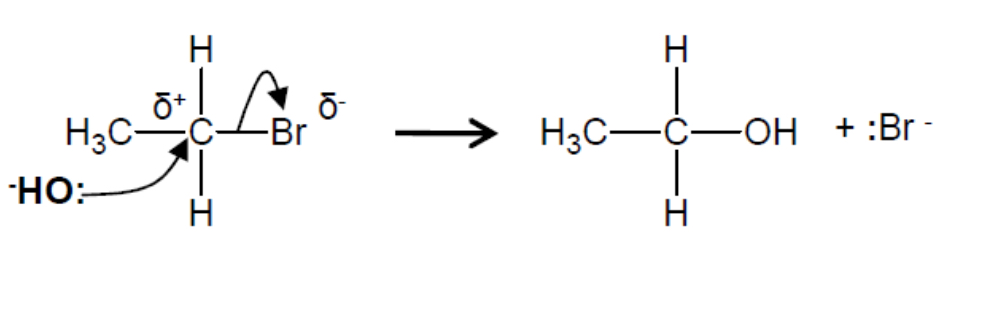
Outline the nucleophilic substitution of bromoethane with a hydroxide ion, including the mechanism
Warm, aqueous conditions with NaOH
The hydroxide ion is a nucleophile (an electron pair donator), so it attacks the polar C-Br bond, forming an alcohol (ethanol) and a bromide radical
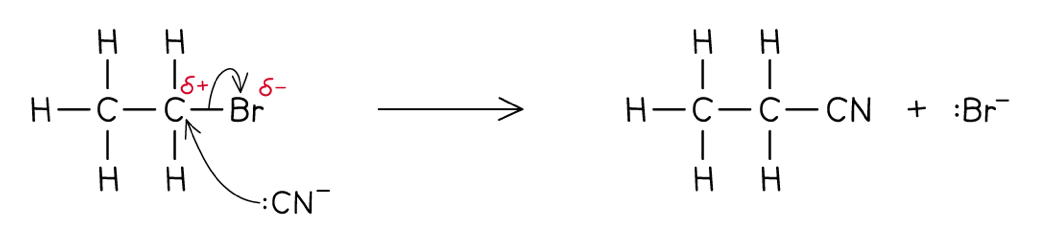
Outline the nucleophilic substitution of bromoethane with a cyanide ion, including the mechanism
The cyanide ion (from hot ethanolic KCN) is a nucleophile (an electron pair donator), so it attacks the polar C-Br bond, forming a bromine radical and a nitrile, which has one more carbon in the chain (propanenitrile)
Outline the nucleophilic substitution of bromoethane with ammonia, including the mechanism
The ammonia (excess, hot, ethanolic, pressure) is a nucleophile (an electron pair donator), so it attacks the polar C-Br bond, forming an amine (ethylamine) and a bromide radical
The reaction is in two parts, as another nucleophile (in this case another ammonia) removes a hydrogen from the NH3+ group
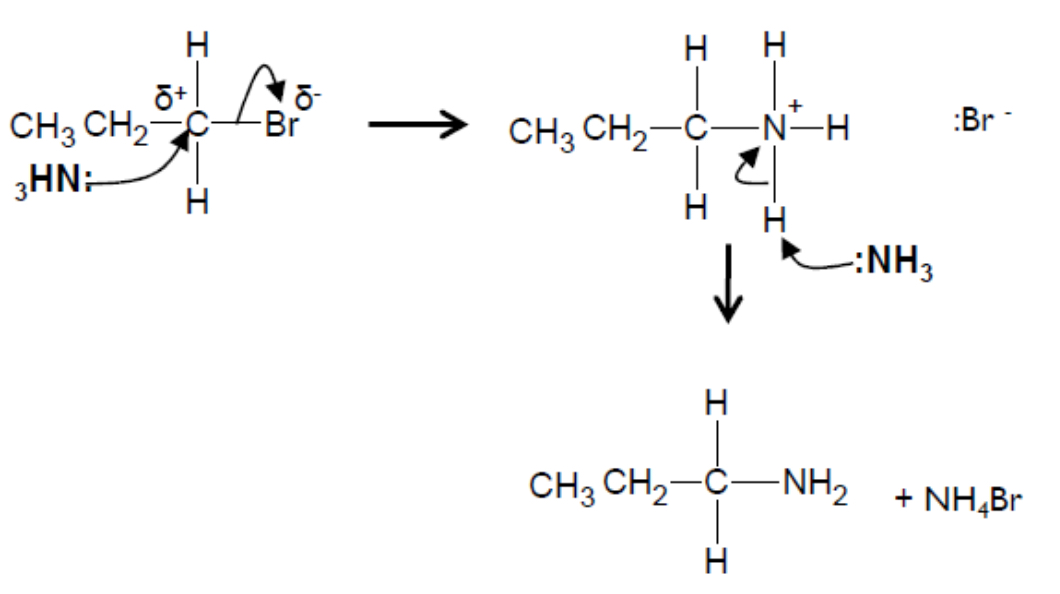
Outline the elimination of 2-bromopropane with a hydroxide ion, including the mechanism
Hot, ethanolic conditions with KOH
The hydroxide ion acts as a base, and bonds with a hydrogen on a neighbouring carbon, so that a C-C double bond is formed and causes the C-Br bond to break, to form an alkene (propene) and a bromide ion

What decides if an elimination or substitution reaction occurs?
Substitution reactions occur in warm aqueous solutions
Primary haloalkanes tend to undergo substitution
Elimination reactions occur in hot ethanolic conditions
Tertiary haloalkanes tend to undergo elimination
In most cases, both will happen to some extent
How can some elimination reactions produce isomers?
Elimination of unsymmetrical secondary or tertiary alcohols can produce two or three structural isomers
This depends on which neighbouring C-C bond becomes a double bond
Why is ozone beneficial?
Ozone (O3) is formed naturally in the upper atmosphere and absorbs UV radiation, which can otherwise cause damages DNA and cause skin cancer
What are CFCs and why were they banned?
CFCs (chlorofluorocarbons) were used industrially as refrigerants, however they were banned because they damage the ozone layer
UV light can break the C-Cl bond in CFCs, which produces a chlorine radical that can catalyse the depletion of ozone
What is the initiation step in the depletion of ozone?
UV light breaks the C-Cl bond in CFCl3 and produces two radicals
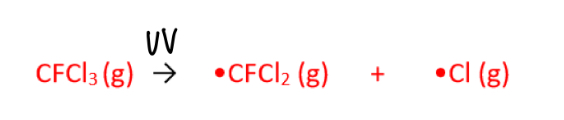
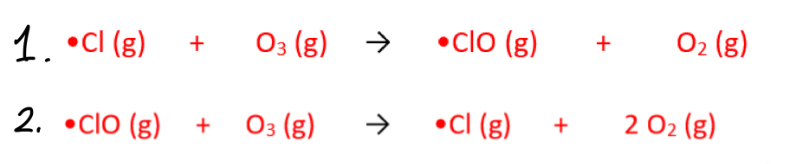
What are the propagation steps for the depletion of ozone?
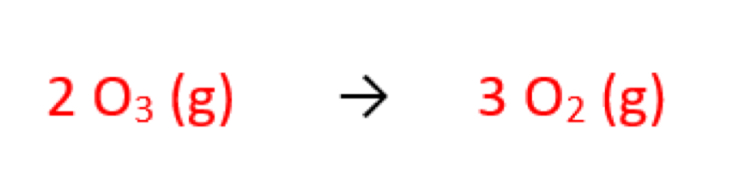
What is the overall equation for the depletion of ozone?
What alternatives to CFCs are being used today and why are they better?
Hydrofluorocarbons (HFCs) are being used as refrigerants instead
They don’t contain the C-Cl bond that can be broken down by UV light to produce a chlorine radical
The C-F bond in HFCs is much stronger