yi 2 recombinant protein purification
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
39 Terms
why is recombinant protein purification necessary
1. many components in cell (proteins, DNA, lipids...)
2. proteins must be pure for medical use (>99%) and research (95-99%)
ex: vaccine, antibody, hormone, crystal formation, immunodetection, restriction enzymes
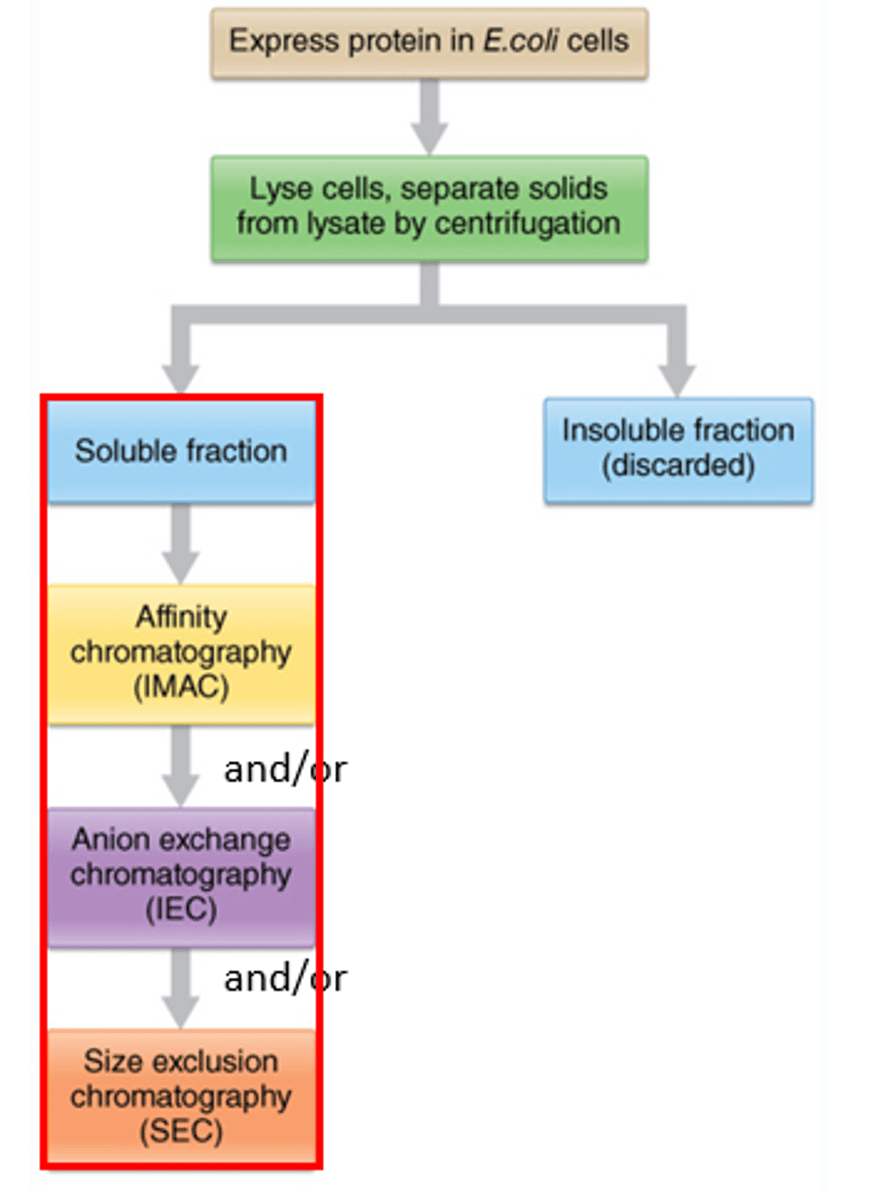
3 methods to extract proteins out of a cell
1. high pressure homogenization
2. sonication
3. lysis using lysis buffers
2 methods to remove particulate matter/contaminants from an extraction
1. centrifugation: separates out the protein
2. filtration: soluble protein passes, debris on top
6 techniques used to separate out small amount of protein
1. SDS-PAGE
2. Isoelectric focusing
3. 2D gel
3. Thin layer chromatography
4. capillary electrophoresis
5. column chromatography----> used in industry
_________________ is suitable for small and large proteins, and industrial scale protein purification
column chromatogrpahy
t/f: column chromatography is only used for small protein separation
false. used for both small and large. industrial scale purification
summarize the 5 types of column chromatography and the protein properties their separation is based on
1. ion exchange= charge
2. hydrophobic interaction= hydrophobicity
3. reversed phase= hydrophobicity
4. gel filtration= size
5. affinity capture= biorecognition
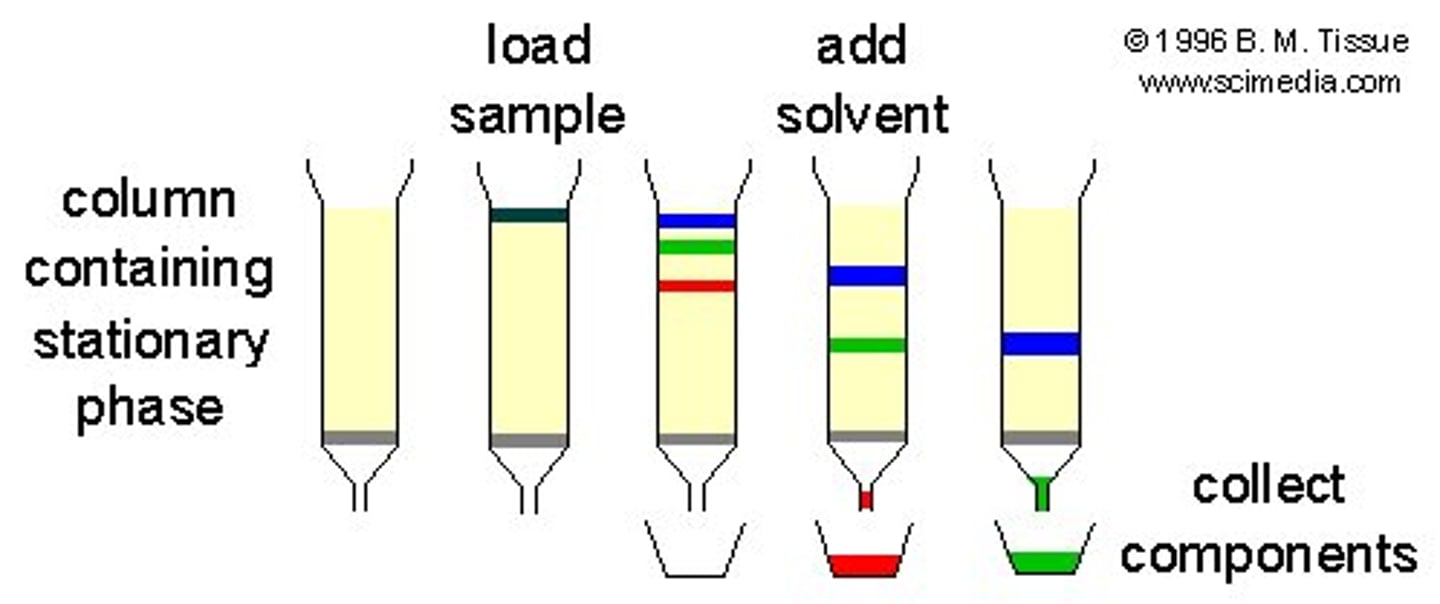
summarize overall steps of a general column chromatography separation
1. proteins are added to the ___________
2. this travels down the ______________ of the column
3. while it travels down the column, a ___________ is added
4. protein fractions are collected
1. proteins are added to the __loading buffer__
2. this travels down the ___resin/matrix/medium____ of the column
3. while it travels down the column, a ____separation buffer____ is added
4. protein fractions are collected
ion exchange chromatography
Basis of separation:
Reversible?
difference in charge
reversible (btwn charged protein and opposite charge of resin)
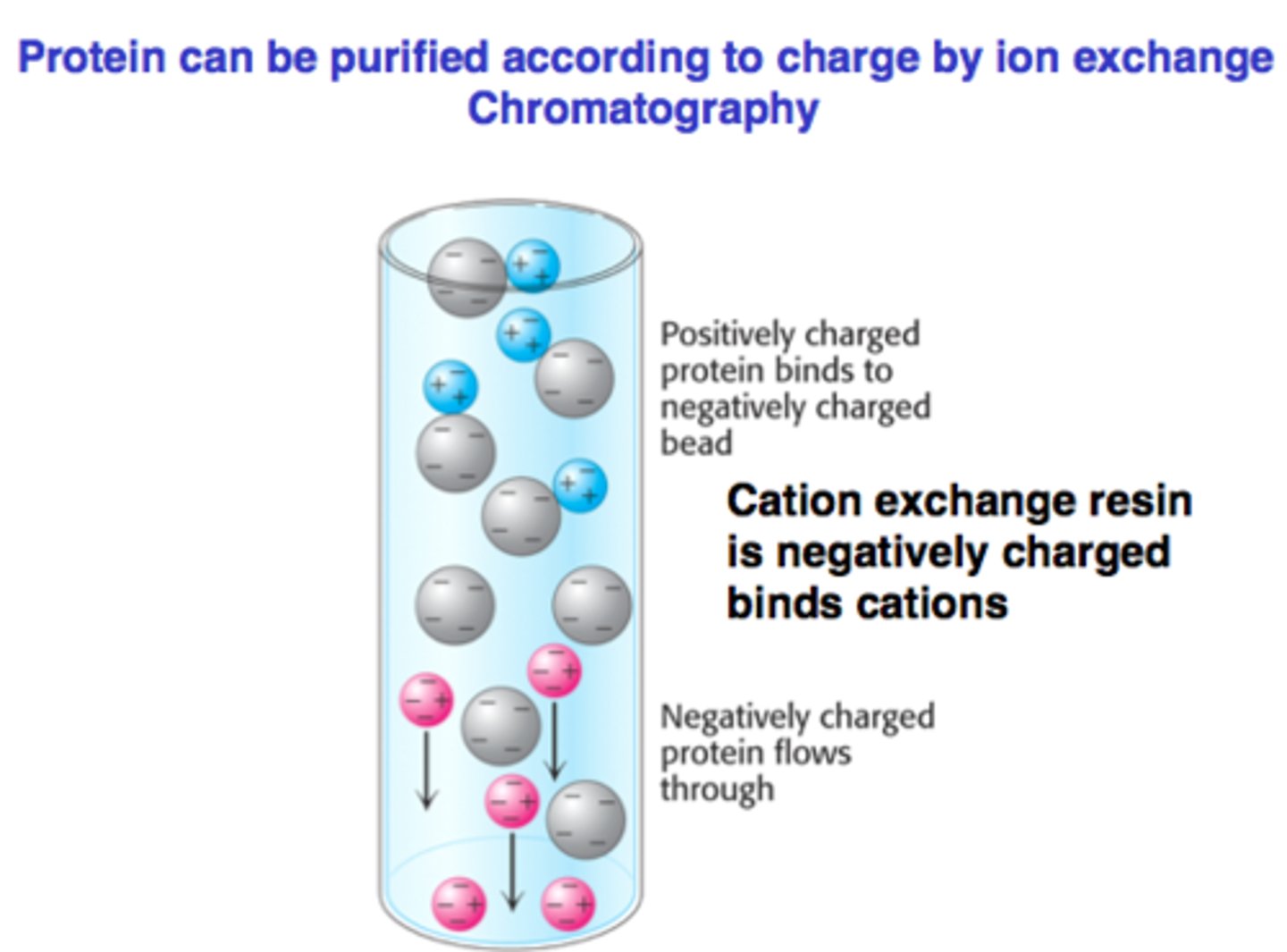
in IEX compare less charge proteins and more charged proteins based on:
1) interaction with charged resin?
2) interaction strength
3) elution
1) interaction with charged resin
-both interact
2) interaction strength
-less charge protein: weaker
-more charge: stronger
3) elution
-less charge: elutes first
-more charge: elutes later (stays on charged resin for longer)
t/f: in IEX, there is an irreversible interaction between charged proteins and the oppositely charged resin
false. reversible
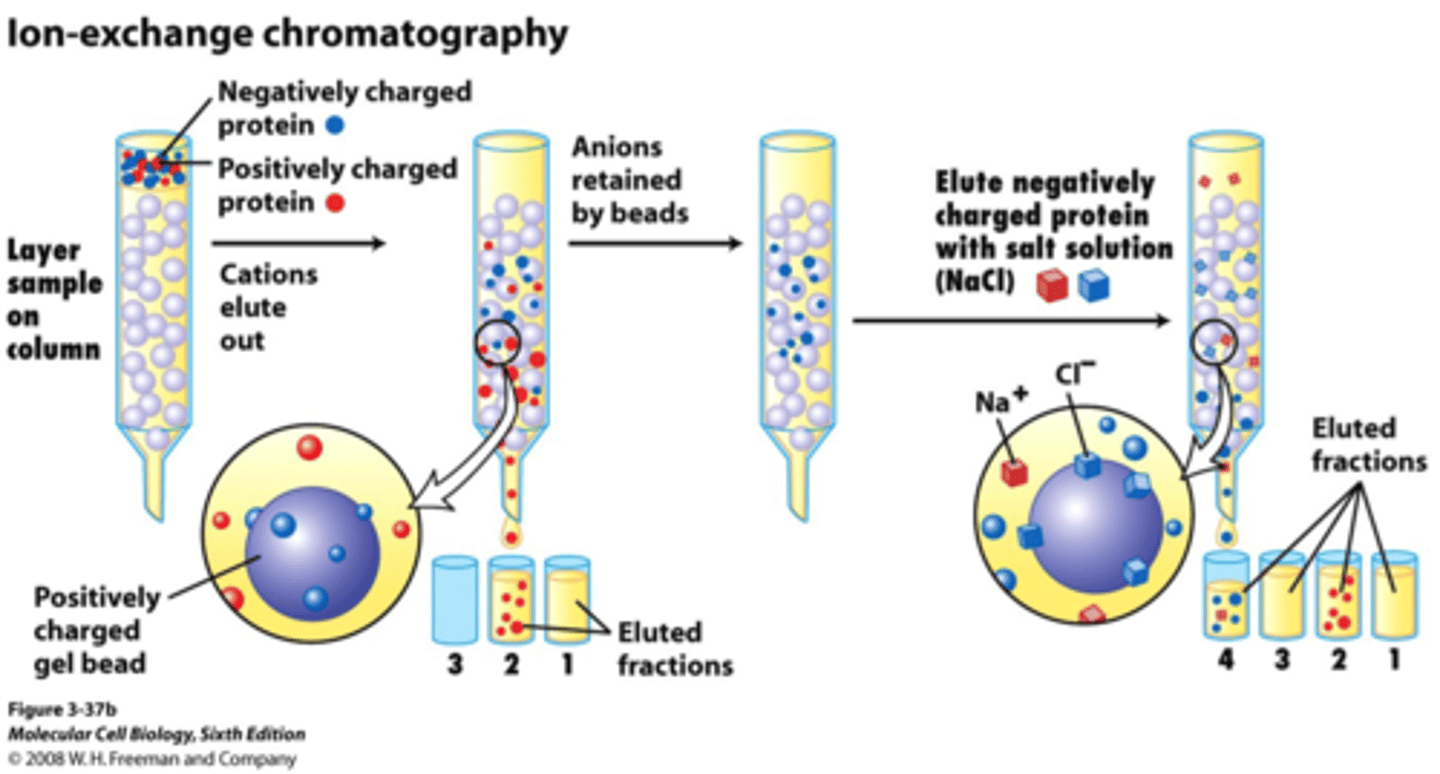
compare cation exchange (CEX) to anion exchange (AEX)
charge of resin?
charge of proteins that interact?
loading pH?
charge of resin:
CEX: neg -
AEX: pos +
charge of proteins that interact:
CEX: pos +
AEX: neg -
loading pH: to compete with protein and kick it off/ same charge as protein
CEX: pH
In CEX, the stationary phase is ________ charged and attracts ________ charged molecules
In CEX, the stationary phase is negatively charged and attracts positively charged molecules
in CEX, the pH is ________ then the pI
less
In AEX, the stationary phase is ____________ charged and attracts ______________ charged molecules
In AEX, the stationary phase is positively charged and attracts negatively charged molecules
in AEX, the pH is _______ then the pI
greater
Steps of IEX:
1. load ______ ionic buffer onto ______ resin
2. separate sample in these 2 ways:
3. which proteins elute first? last?
1. load LOW ionic buffer onto CHARGED resin
2. Separation: increase salt concentration or change pH
3. less charged elute first, more charged elute last
in IEX, why do we start with a low ionic buffer? why do we increase salt concentration?
1. start with low ionic buffer so that the protein binds to resin and there is no competition with NaCl
2. increase [NaCl] so that it begins to compete with proteins and elute them out
hydrophobic interaction chromatography
basis of separation?
reversible?
- differences in hydrophobicity
- reversible
in HIC compare less hydrophobic proteins and more hydrophobic proteins based on:
1) interact with hydrophobic resin?
2) interaction strength
3) elution
1. resin intrxn: both
2. strength
less: weaker
more: stronger
3.elution
less: first
more: later
ideal next steps after HIC
1. sample precipitation with ammonium sulfate
2. elution in high salt during IEX
Steps of HIC:
1. load ______ ionic buffer onto ______ resin
2. separate sample by:
3. which proteins elute first? last?
Steps of HIC:
1. load HIGH ionic buffer onto SMALL HYDROPHOBIC resin
2. separate sample by: DECREASING salt concentration
3. LESS hydrophobic= first
MORE hydrophobic= last
in HIC, explain why we start with a high ionic buffer and decrease salt concentration
Begin with high ionic buffer (ammonium sulfate) so that the hydrophobic proteins attach to hydrophobic resin (if you start with low ionic buffer then theres less of a difference and they may wash out)
Gradually decrease the salt concentration so that the hydrophobic proteins are more likely to wash out with the less charge buffer
which buffer is used in HIC
ammonium sulfate (salt starts out high then is reduced)
RPC vs HIC
resin surface:
interaction strength with proteins:
loading buffer:
separation buffer:
resin surface:
RPC: large hydrophobic
HIC: small hydrophobic
interaction strength with proteins:
RPC: strong
HIC: Weak
loading buffer:
RPC: low hydrophobicity
HIC: high ionic
separation buffer:
RPC: increase in hydrophobic solvent (acetonitrile, methanol)
HIC: decrease salt concentration
separation buffers of RPC
acetonitrile and methanol (hydrophobic solvents)
Steps of RPC:
1. load ______ buffer onto ______ resin
2. separate sample by:
3. which proteins elute first? last?
Steps of RPC:
1. load LOW HYDROPHOBIC buffer onto LARGE HYDROPHOBIC resin
2. separate sample by: increasing hydrophobicity (acetonitrile/methanol)
3. less hydrophobic out first, more hydrophobic out last
gel filtration (GF) is also known as
size exclusion chromatography (SEC)
gel filtration chromatography
Basis of separation:
Interaction with resin?
- difference in size
- NO interaction with resin
in GF compare large proteins and small proteins based on
1) interact with resin?
2) enter pores?
3) elution
1) interact with resin?
-NO interaction with either
2) enter pores?
large: no
small: yes
3) elution
large: first
small: last
t/f: gel filtration uses a single buffer for separation
true. interaction is not based on charge so the buffer is not changed
t/f: gel filtration uses a small sample volume
true
describe the steps of a typical GF separation
1. load any suitable buffer and your proteins
2. small proteins get stuck in the pores of the resin while large ones bypass them and elute out
3. continue adding buffer (do not change it!) until small proteins get out too
affinity capture (AC) chromatography
Basis of separation:
Interaction with resin?
- difference in affinity/biorecognition
- REVERSIBLE interaction btwn protein and ligand on resin
in AC compare no affinity proteins and affinity proteins based on:
1) interact with resin?
2) interaction strength?
3) elution
1) interact w resin?
no affinity: no
affinity: yes
2) intrxn strength:
no affinity: none
affinity: high
3) elution
no affinity: first
affinity: later
In AC, which resins are used for the following proteins?
1. His-tagged
2. GST- tagged
3. Flag/Myc/V5-tagged
4. antibody
1. His-tagged
= metal-chelated (nickel and cobalt)
2. GST- tagged
= glutathione-conjugated
3. Flag/Myc/V5-tagged
= anti Flag/Myc/V5-tagged antibody conjugated
4. antibody
= ProteinA/G conjugated
t/f: one main reason for introducing fusion tags onto proteins is to separate them in size exclusion chromatography
false. affinity capture chromatography
Steps of AC:
1. load ______ buffer onto ______ resin
2. separate sample by:
3. which proteins elute first? last?
1. load buffer with affinity proteins to resin w ligand
2. separate: add competitive ligand, change pH, increase salt/organic solvent
3. no affinity= first
high affinity= last
t/f: a competitive ligand may be added to AC to separate high affinity proteins
true