Chap 4C - chemical bonding
1/23
Earn XP
Description and Tags
Intermolecular forces of attraction
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Define dipole + SI unit
Def. : A dipole refers to a separation of positive and negative charges within a molecule or atom caused by uneven distribution of electrons resulting in a region with a partial positive charge (δ+) and a region with partial negative charge (δ−) leading to formation of a polar molecule
Strength of dipole measured by magnitude of dipole moment
Unit : Debye (D)
Describe the 2 types of dipole
Permanent dipole (Eg. HCI)
Occurs in polar molecules, where atoms have different electronegativities, causing an uneven distribution of electrons
Induced dipole (Eg. O2)
Occurs in non-polar molecules, where a temporary dipole can be induced in a molecule by a nearby permanent or temporary dipole
Define intermolecular forces of attraction
Intermolecular forces of attraction (Def.) : It is the attraction between molecules. These forces are generally weaker than covalent, ionic or metallic bonds but play a crucial role in determining the physical properties of substances, such as boiling points, melting points, solubility, and volatility.
Define dispersion forces
Def. : These are weak, temporary electrostatic attractions or interactions that arise from the momentary uneven distribution of electrons in atoms or molecules, creating instantaneous dipoles
Describe dispersion forces
Between nonpolar or polar substances
Dispersion forces exist between all molecules or between noble gas atoms, only IMF in nonpolar molecules
Weakest among IMFs but becomes larger, more polarisable molecules
Comparison is made between molecules of comparable number of electrons
Explain how dispersion forces are formed
The average electron density distribution of a non–polar molecule is symmetrical
However, as electrons are in constant, random motion, the electron cloud distribution of the molecule may appear asymmetrical/distorted at a given instant, giving rise to an instantaneous dipole
The instantaneous dipole then proceeds to polarise/distort the electron cloud of an adjacent molecule, giving rise to an induced dipole
The resulting electrostatic forces of attraction between these two dipoles is known as instantaneous dipole–induced dipole interactions (forces of attractions)
Short-lived as electrons continue to be constant, random motion, causing the dipoles to vanish and reappear intermittently
Describe the factors of dispersion forces
The number of electrons in the molecule
The larger the number of electrons, the larger and more easily polarisable the electron cloud, the stronger the dispersion forces
Larger the Mr, greater the number of electrons in the molecule
The surface area of contact between adjacent molecules
The larger the surface area of contact, the more easily induced dipoles are formed, the stronger the dispersion forces
Straight-chain or linear molecules have greater surface area than branched molecules (more compact and spherical)
Eg. Pentane has an elongated shape and larger surface area than 2,2-dimethylpropane which has a spherical shape
Define permanent dipole-permanent dipole attractions
(Def.) : These forces or interactions or attractions occur between molecules that have permanent dipoles (polar molecules), where the positive end of one dipole is attracted to the negative end of another
Describe permanent dipole-permanent dipole attractions
Between polar molecules
Stronger than id-id forces of comparable Mr but weaker than hydrogen bonds
Become stronger with increasing polarity of molecule
Explain how permanent dipole-permanent dipole attractions is formed
Polar molecules have permanent dipoles in their structures
These molecules tend to align themselves such that the partially positive (+) end of one molecule is near the partially negative (–) end of another molecule
The electrostatic forces of attraction between the + end of one molecule and the – end of another molecule is permanent dipole–permanent dipole interactions
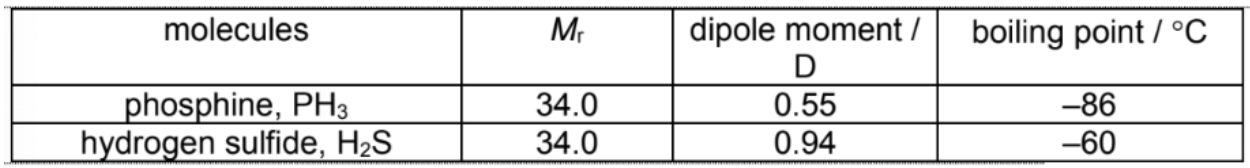
Explain the trend in boiling points of these molecules
Both phosphine and hydrogen sulfide are polar molecules held by permanent dipole–permanent dipole (pd–pd) interactions and instantaneous dipole-induced dipole (id-id) interactions. The strength of their id-id interactions is comparable as they have a comparable number of electrons (same Mr). H2S is more polar (larger dipole moment) than PH3 and so the pd-pd interactions between H2S are stronger. Hence, more energy is required to overcome the attractive forces between H2S molecules leading to a higher boiling point.
Define hydrogen bonding
|
State the 2 conditions for hydrogen bonds to form
The molecule must contain a hydrogen atom bonded to a highly electronegative atom (F, O, N)
There must be an atom (F, O, N) with a lone pair of electrons
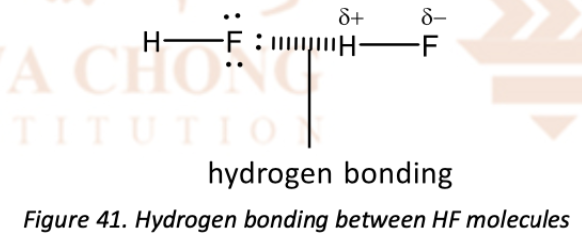
Explain how hydrogen bonds are formed using HF
The bonding electrons in H–F bond are pulled strongly towards the highly electronegative fluorine atom
The hydrogen atom (which has no inner electrons is practically left with its nucleus with one proton) is highly electron deficient (carries a significant + charge).
The lone pairs on F, found in 2p orbitals which are small, is able to attract the electron deficient H atom strongly
Draw the diagram of hydrogen bonding (using HF)
Label the dipole for the covalent bond (F–H, O–H or N–H) carrying the acceptor hydrogen atom with '+' symbol on H and '–' symbol on the more electronegative F or O or N atom
Draw the lone pair of electrons for the fluorine, oxygen or nitrogen atom involved in the hydrogen bonding
Indicate the hydrogen bonding
Describe the factors of hydrogen bonding
Extent of hydrogen bonds
Depends on number of H-X bonds and number of lone pairs available on the highly electronegative atom
The greater the extent of hydrogen bonds, the stronger the attractions between molecules and so more energy is needed
Eg. HF : 1 hydrogen bond per molecule, H2O : 2 hydrogen bonds per molecule, NH3 : 1 hydrogen bond per molecule
Electronegativity of atom that H atom is bonded to
For example, F is more electronegative than N. The + charge on H in HF is larger than that in NH3 hence the hydrogen bonding between HF molecules is stronger
Polarity of H-X bond
H–X bond with larger dipole moment is more polar resulting in stronger hydrogen bonds.
Bond polarity : H–F > H–O > H–N
Explain why hydrogen bonding in H2O is more extensive than that in HF or NH3
H2O has two lone pairs of electrons and two electron deficient hydrogen atoms -> can form on average two hydrogen bonds per molecule
NH3 has only one lone pair (despite having three hydrogen atoms) , HF has only one electron deficient hydrogen atom (despite having three lone pairs) -> NH3 and HF can only form one hydrogen bond per molecule
Explain why the boiling point of HF (20 C) is higher than NH3 (–33 C)
Both are highly polar molecules held by hydrogen bonding. The extent of hydrogen bonds is the same for both HF and NH₃ since both form one hydrogen bond per molecule. However, the H–F bond is more polar than the H–N bond since F is more electronegative than N, resulting in stronger hydrogen bonds between HF molecules than those between NH₃ molecules. Hence, more energy is required to overcome the stronger attractive forces between HF molecules, leading to its higher boiling point than NH₃
Explain how hydrogen bonding causes higher than expected boiling and melting points for NH3, H2O and HF
Description :
Boiling points of Group 15 hydrides increase from PH3 to SbH3
The same trend is also observed for Group 16 hydrides from H2S to H2Te and Group 17 hydrides from HCl to HI
There is an anomaly in the boiling point of the first hydride in each group
Explanation : (For group 15)
All group 15 molecules are polar
Ammonia has an unusually high boiling point despite having the smallest molecular size compared to the other Group 15 hydrides
Due to hydrogen bonding -> N is highly electronegative -> highly polar N-H bond which allows ammonia molecules to form strong hydrogen bonds -> these hydrogen bonds require more energy to break during boiling
For the heavier hydrides, PH3 to BiH3, they are unable to form hydrogen bonds because phosphorus, arsenic, antimony, and bismuth are less electronegative than nitrogen.
These molecules experience both permanent dipole-permanent dipole (pd-pd) and instantaneous dipole–induced dipole (id-id) forces
The boiling point increases down the group due to the increasing number of electrons -> larger and more easily polarised electron cloud -> stronger id-id forces, requiring more energy to overcome
Explain why boiling point of 2–nitrophenol is lower than 4–nitrophenol even though both have the same relative molecular mass due to intramolecular hydrogen bonds [4 marks]
Both compounds have simple molecular structures with instantaneous dipole-induced dipole (id-id) interactions and hydrogen bonds between the molecules.
Since both have the same number of electrons, the difference in their boiling point is caused by the extent of the hydrogen bonds rather than the strength of the id-id forces.
The proximity of –OH and –NH2 groups next to each other in 2-aminophenol allows them to form intramolecular hydrogen bonds -> lesser extent of intermolecular hydrogen bonding resulting in a lower boiling point
The –OH and –NH2 groups in 4-aminophenol are too far apart to allow for the formation of intramolecular hydrogen bonds -> greater extent of intermolecular hydrogen bonding resulting in a higher boiling point
Compare intermolecular and intramolecular hydrogen bonds
Intermolecular hydrogen bonding occurs between molecules, increasing boiling points and creating strong intermolecular forces.
Intramolecular hydrogen bonding occurs within the same molecule, reducing the extent of intermolecular interactions
Describe dimerization of carboxylic acids
In non-polar solvents (benzene), carboxylic acids like CH₃CO₂H form dimers through hydrogen bonding, where two molecules bond together
This dimerization doubles the observed molecular mass (Mr) to 120 (instead of the expected 60).
In polar solvents like water, carboxylic acids form hydrogen bonds with water molecules instead of dimerizing
Explain why ice is less dense than water even tho ice is a solid
Liquid |
|
Solid |
|
As ice melts |
|
Explain why water has a higher than expected surface tension due to dimerisation
Surface tension (Def.) : Amount of force required to break the layer of water molecules on its surface
The molar mass of some carboxylic acids in vapour phase or in non–polar solvents is found to be twice its molar mass as calculated from its molecular formula
In vapour phase or in non–polar solvents, some carboxylic acids tend exist as dimers, whereby two carboxylic acid molecules form hydrogen bonds with each other
In polar solvent such as water, dimerisation of carboxylic acids is less likely to occur due to the higher tendency of carboxylic acid molecules to form hydrogen bonds with water molecules rather than its own molecules