SAR, Drug Discovery, and Development
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
chemotype
-The type of a drug passed on a prominent chemical substructure(s) contained within it
-Defines the overall chemical or structural class(es) of a therapeutic agent
-May be fully or partially responsible for pharmacological activity, or not at all
pharmacophore
-Defines the structural elements necessary for pharmacological activity (i.e., potent and productive interaction with the target)
-Usually includes a core structure with key functional groups
SAR (structure-activity relationship)
an evaluation of how changes in molecular structure correlate with changes in target activity; the relationship between the chemical structure of a drug and its physicochemical, pharmacological, and therapeutic activities
(Q)SPR (structure-property relationship)
describes how changes in molecular structure correlate with changes in a physical property such as solubility or lipophilicity
pharmacore-based (ligand based)
approach to drug design that starts with molecule with known activity of natural origin and try to modify it; create analogs and test to try to mimic activity of natural product
target-based (receptor/site-based)
approach to drug design that focuses on the structure of the target through X-ray crystallography or NMR
conformational restriction
functional group variation
isosteric modification
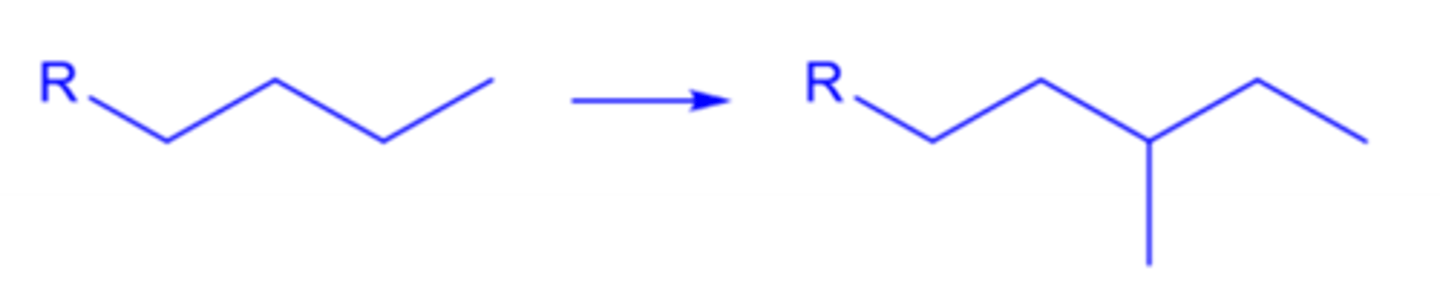
homologation and branching
four basic concepts of molecular modification
conformational restriction
Can involve:
-Substitution of adjacent rings with bulky groups can force one ring to rotate out of plane of the other one. This can be more favorable for during drug binding to its target or preventing a drug from being destroyed by an enzyme (ex: diclofenac)
-Replacing a single bond with a double bond can restrict rotation and force a molecule to adopt a specific stereochemistry which enhances receptor binding (ex: chlorpromazine)
functional group variation
Can involve:
-Replacing a polar group with a lipophilic group can enhance oral bioavailability and/or pharmacological activity
(Ex: Clindamycin)
-Removal of a certain functional group can inhibit metabolic enzymes crucial to the survival of viruses
(Ex: dideoxy-antiviral)
-Substitution of readily metabolizable functional group with a more stable one can extend duration of action of certain drugs
(Ex: lidocaine)
isosteric modification
molecular modification in which groups may be interchanged to generate synthetic analogs that can be defined as isosteres; may be classified as classical (follow simple rules) or non-classical (deviate from simple rules) but both are employed in developing drug candidate analogs for many diseases conditions
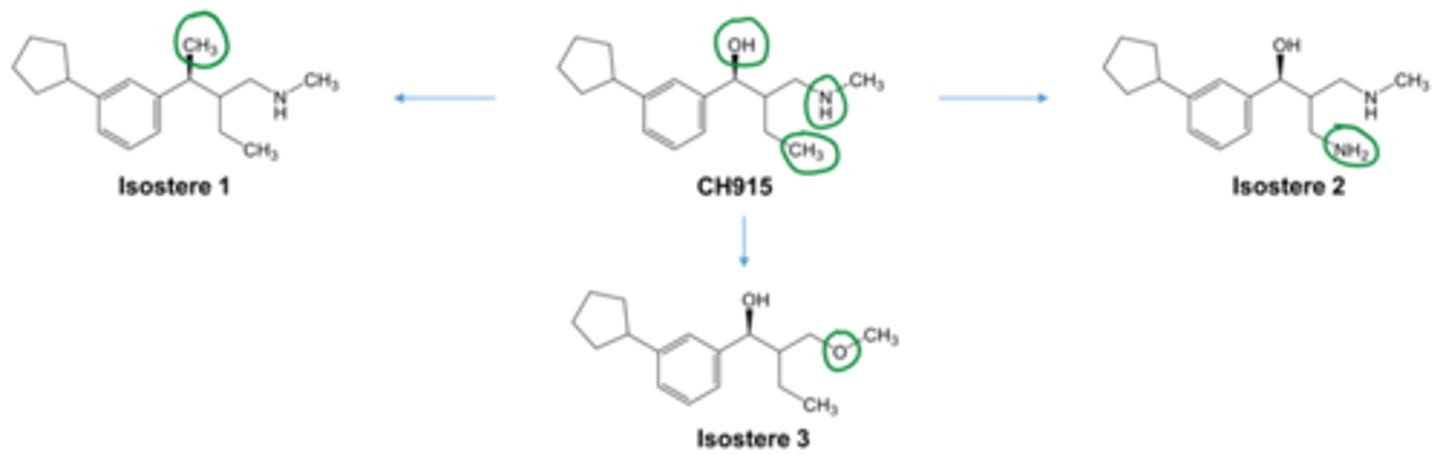
isosters
compounds or groups of atoms that have the same number and arrangement of outer electrons
Ex: O = CH2 = NH = 8 electrons
bioisosteres
functional groups or molecules that have similar chemical and physical properties that produce broadly similar biological properties
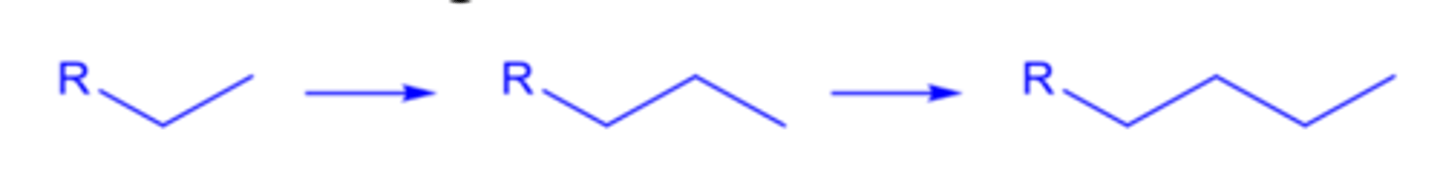
homologation
successively adding methylene groups (-CH2-) to a hydrocarbon chain
chain branching
insertion of methyl groups or moving functional groups in a linear hydrocarbon chain to introduce branching