Biochem Ch. 7 Enzyme Kinetics & Regulation
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
21 Terms
first-order reaction
the velocity of a reaction is directly proportional to reactant concentration V=k[A]
second-order reaction
rate depends on two reactants colliding, doubling would equal in 4x speed (ex: dance floor where two people collide) V=k[A]2 or V=k[A][B]
initial velocity (Vo)
the early reaction rate measured before substrate drops or product accumulates—giving the purest snapshot of enzyme activity
KM
= k-1+k2 / k1
What does KM indicate?
the substrate binding with the enzyme
a high KM
low binding
a low KM
high binding
dissociation constant
the concentration of ligand needed to occupy half of the binding sites, and it directly reflects how tightly two molecules bind (ex: how tightly you hold a balloon and how often it gets away)
turnover number
K2 or kcat, the number of substrate molecules converted into product per second
Michaelis-Menten equation
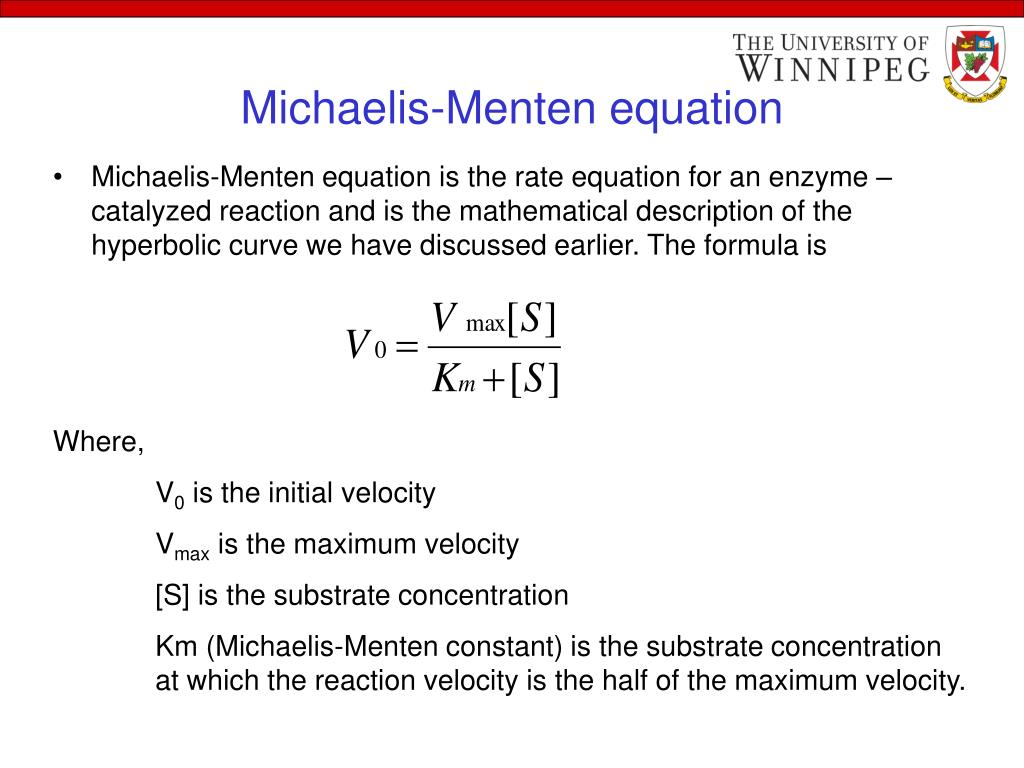
Vmax (kcat)
top speed of the enzyme, can’t work any faster
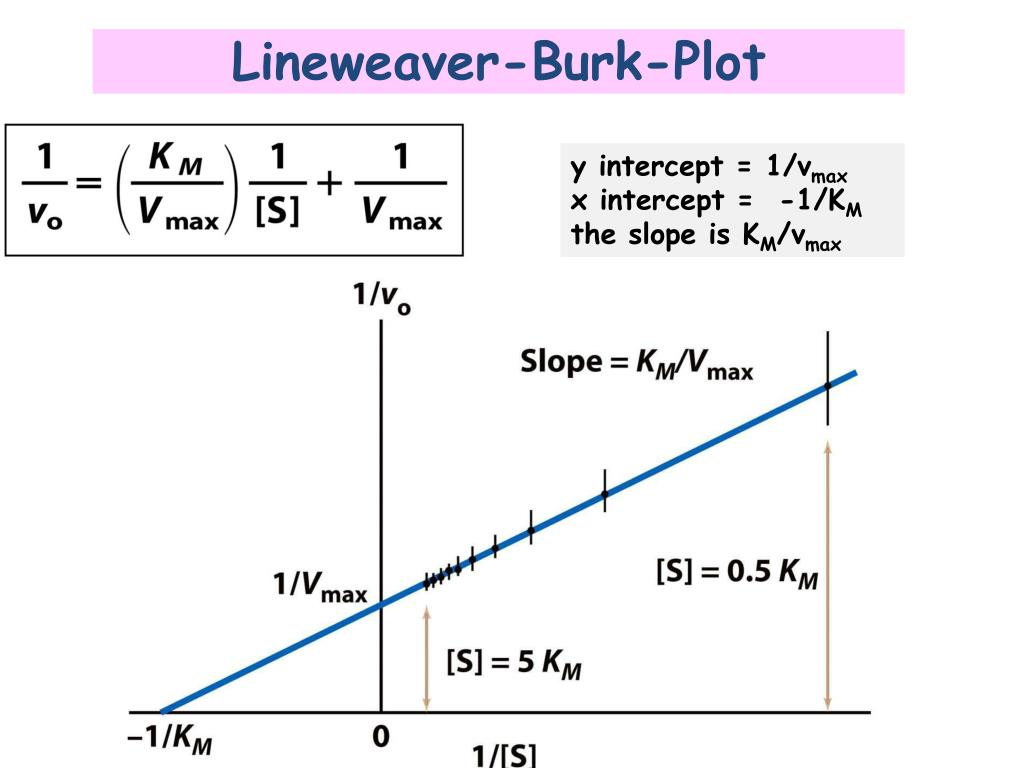
Lineweaver-Burk equation
double-reciprocal equation, turns curved Michaelis-Menten graph into a straight line
allosteric control
a molecule binds to a site on an enzyme other than the active site and changes the enzyme’s activity
aspartate transcarboxylase (ATCase)
catalyzes the first step in pyrimidine synthesis
feedback inhibition
ATCase is inhibited by the end product of the pathway, CTP
How are allosterically regulated enzymes different kinetically?
sigmoidal curve
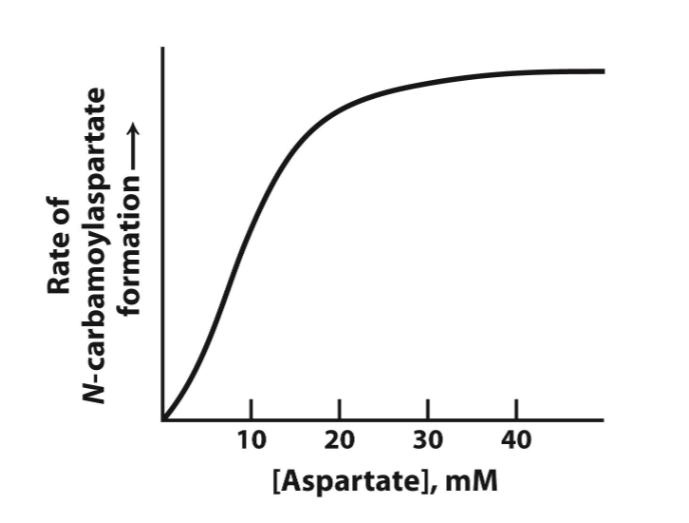
threshold effect
small change in substrate concentration has significant impact
How are allosteric interactions in ATCase mediated?
by large changes in quaternary structure
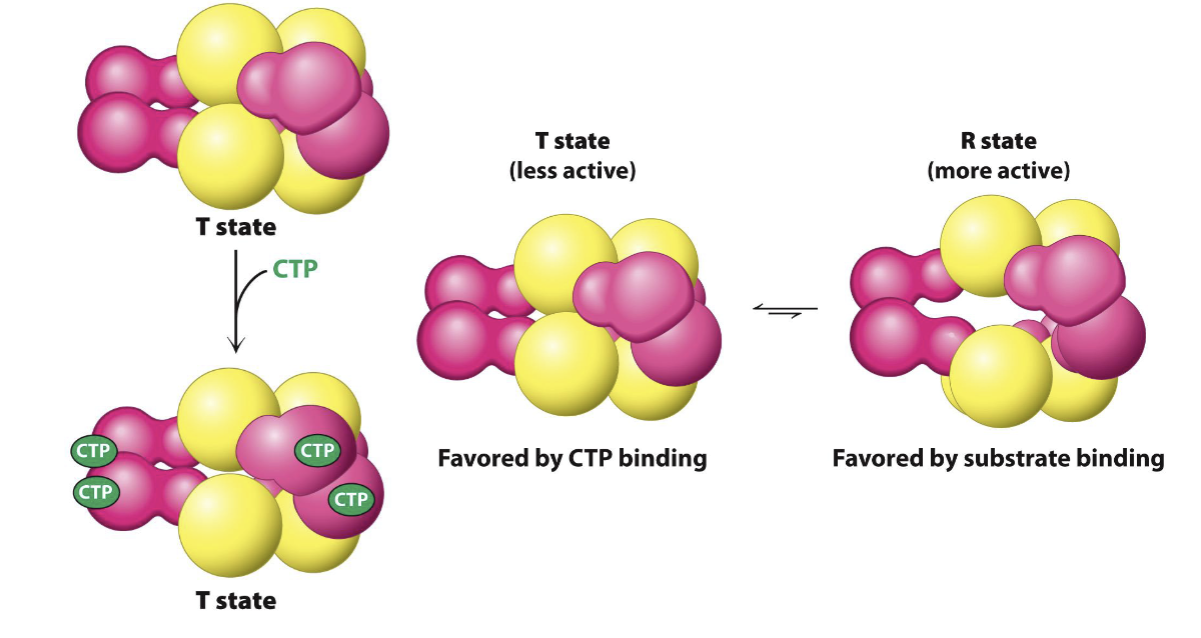
What are the parts of a catalytic trimer?
regulatory / r chain, catalytic / c chain, and regulatory dimer
ATCase T-state
lowers enzyme activity, favored by CTP binding
ATCase R-state
more relaxed, opens up, favored by substrate binding