Core Practical 7: Identify unknown organic liquids and inorganic solids
0.0(0)
Card Sorting
1/7
There's no tags or description
Looks like no tags are added yet.
Last updated 11:53 AM on 1/7/26
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
1
New cards
Overview:

2
New cards
Procedure (Part 1):

3
New cards
Procedure (Part 2):

4
New cards
Which functional groups are in compounds A, B and C? Be as specific as possible.
5
New cards
Identify the inorganic solids X, Y and Z.
6
New cards
State the type of organic liquid product that forms for any of the reactions that occur during tests 1 to 3 in Part 1.

7
New cards
There are a lot of reactions going on in Part 2, test 2 (a,b and c). Write ionic equations for these reactions for the anions in X, Y and Z.
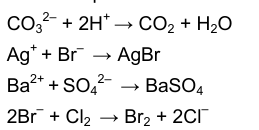
8
New cards
Why is the nitric acid added in the test for halide ions using silver nitrate.
To react with any carbonate ions so that a ppt of silver carbonate does not form.