NUCLEAR PHYSICS TO REMEMBER (finish)
1/100
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
101 Terms
Nucleon
A proton or neutron in the nucleus of an atom
Nucleon number / Mass number
The number of protons and neutrons in the nucleus of an atom
Proton number / Atomic number
The number of protons in the nucleus of an atom
Isotope
An atom of the same element (i.e. same number of protons) but with a different number of neutrons
Rutherford scattering - what was it + objective
- passes beams of alpha particles through a thin sheet of gold foil to investigate:
1. the angles of deflection of alpha particles
2. the number of alpha particles that were deflected at each angle
Rutherford scattering - findings + what each suggested
1. the majority of α-particles passed straight through the foil undeflected
- suggesting that the atom is mostly empty space
2. some α-particles deflected through small angles of <10°
- suggesting there is a positive nucleus at the centre
3. only a small number of α-particles deflected straight back at angles of >90°
- suggests that the nucleus is extremely small + its where most of the mass & charge of the atom is concentrated
→ ATOMS consist of SMALL, DENSE, POSITIVELY CHRAGED NUCLEI SURROUNDED BY NEGATIVELY CHARGED e⁻
SYNOPSIS OF CHANGING MODELS OF NUCELUS
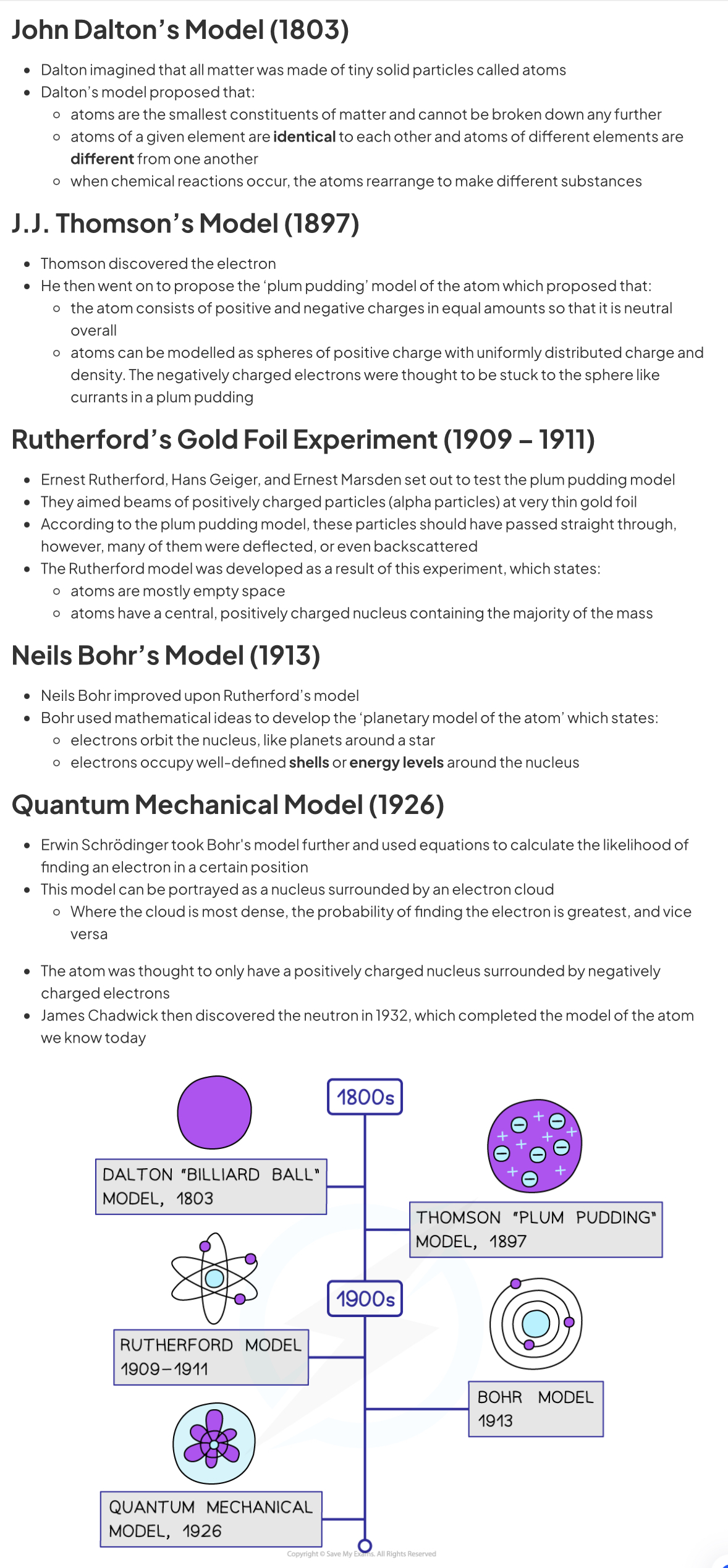
Alpha particle
Two protons and two neutrons. Alpha decay results in proton number decreasing by 2, and nucleon number decreasing by 4
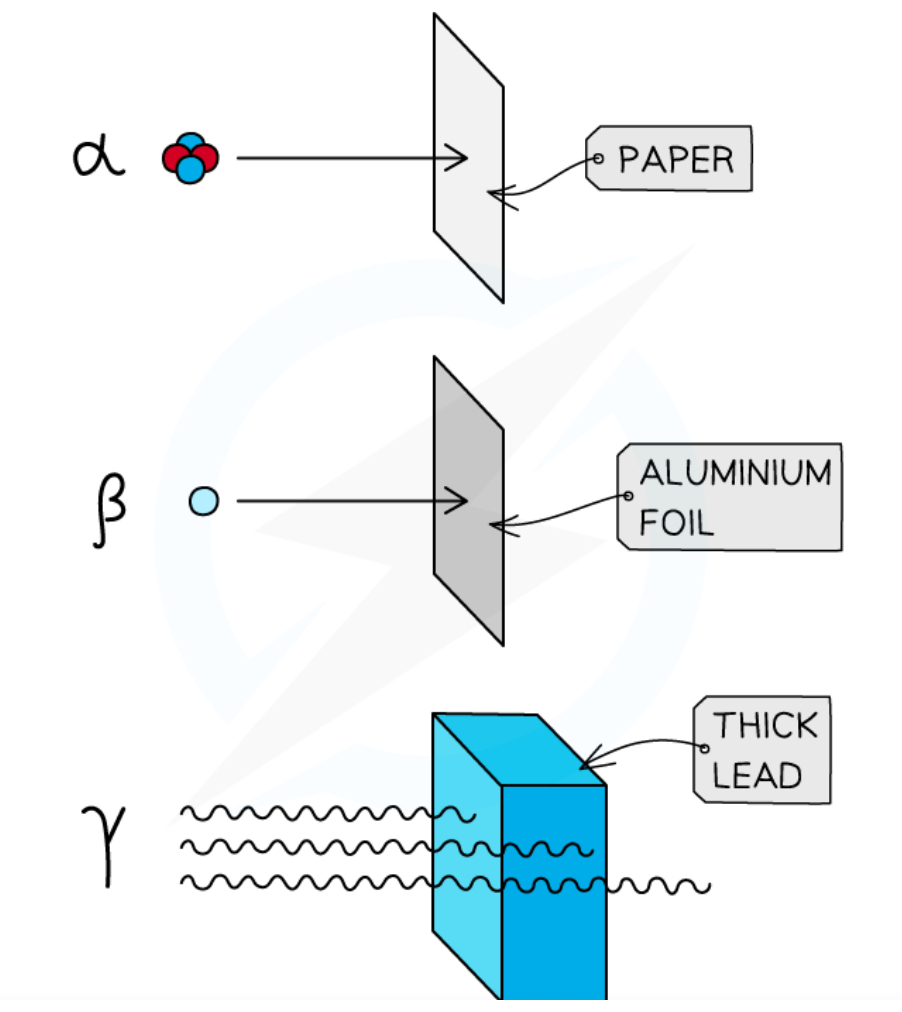
Properties of alpha particles
- 2 protons + 2 neutrons, 4u mass, +2e charge
- most ionising radiation cos they have the greatest charge
- least penetrating → stopped by 3 to 7 cm in air
- absorbed by paper
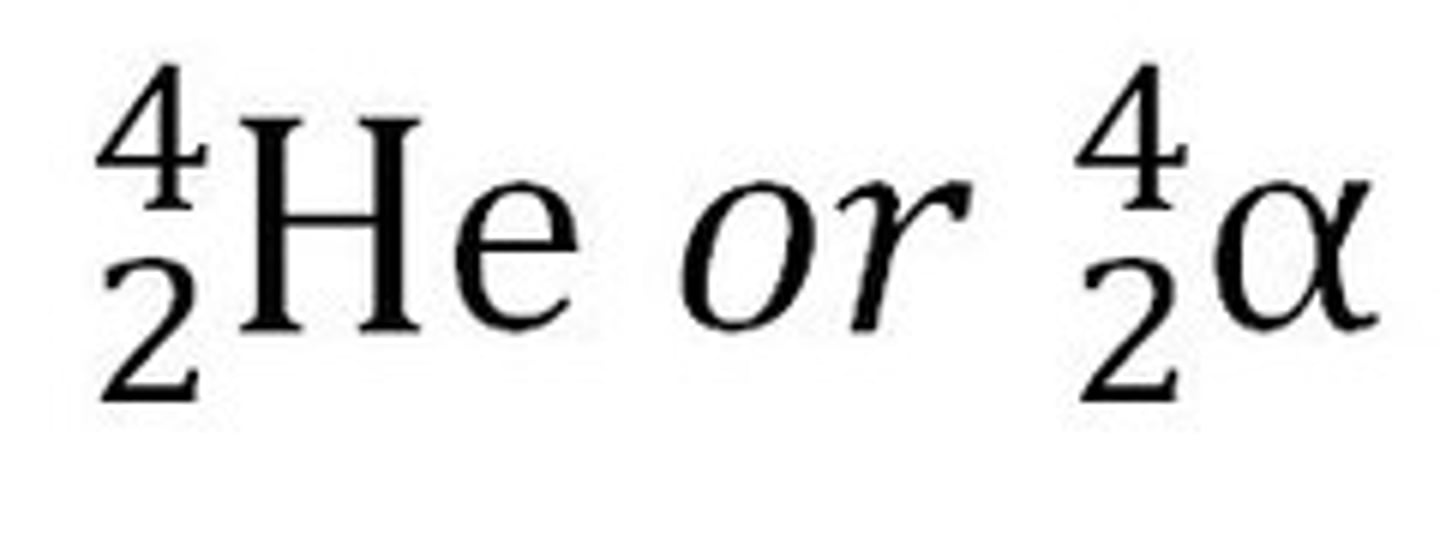
Beta (minus) particle
An electron. In beta (minus) decay, a fast moving electron and an antineutrino are emitted from the nucleus. Beta minus decay results in proton number increasing by 1 and nucleon number remaining the same
Beta (plus) particle
A position (anti electron). In beta (plus) decay, a fast moving positron and a neutrino are emitted from the nucleus. Beta plus decay results in proton number decreasing by 1 and nucleon number remaining the same
Properties of beta particles
- beta-minus (β−) is emitted by nuclei w/ too many neutrons
AND
- beta-plus (β+) is emitted by nuclei w/ too many protons
- moderately ionising
- moderately penetrating → stopped by around 20 cm to 3 m in air
- absorbed by aluminium foil ~ 3mm

Gamma radiation
High frequency e-m radiation emitted by an unstable nucleus
Properties of gamma particles
- emitted by nuclei that need to lose some energy
- least ionising cos they have no charge
- most penetrating, has an infinite range (follows inverse square law)
- absorbed by thick lead or concrete
Inverse-square law for γ radiation
The moment the light leaves the source, it begins to spread out uniformly as a sphere
ONLY APPLIES TO GAMMA bc its not absorbed by matter easily

Experimental verification of inverse-square law?
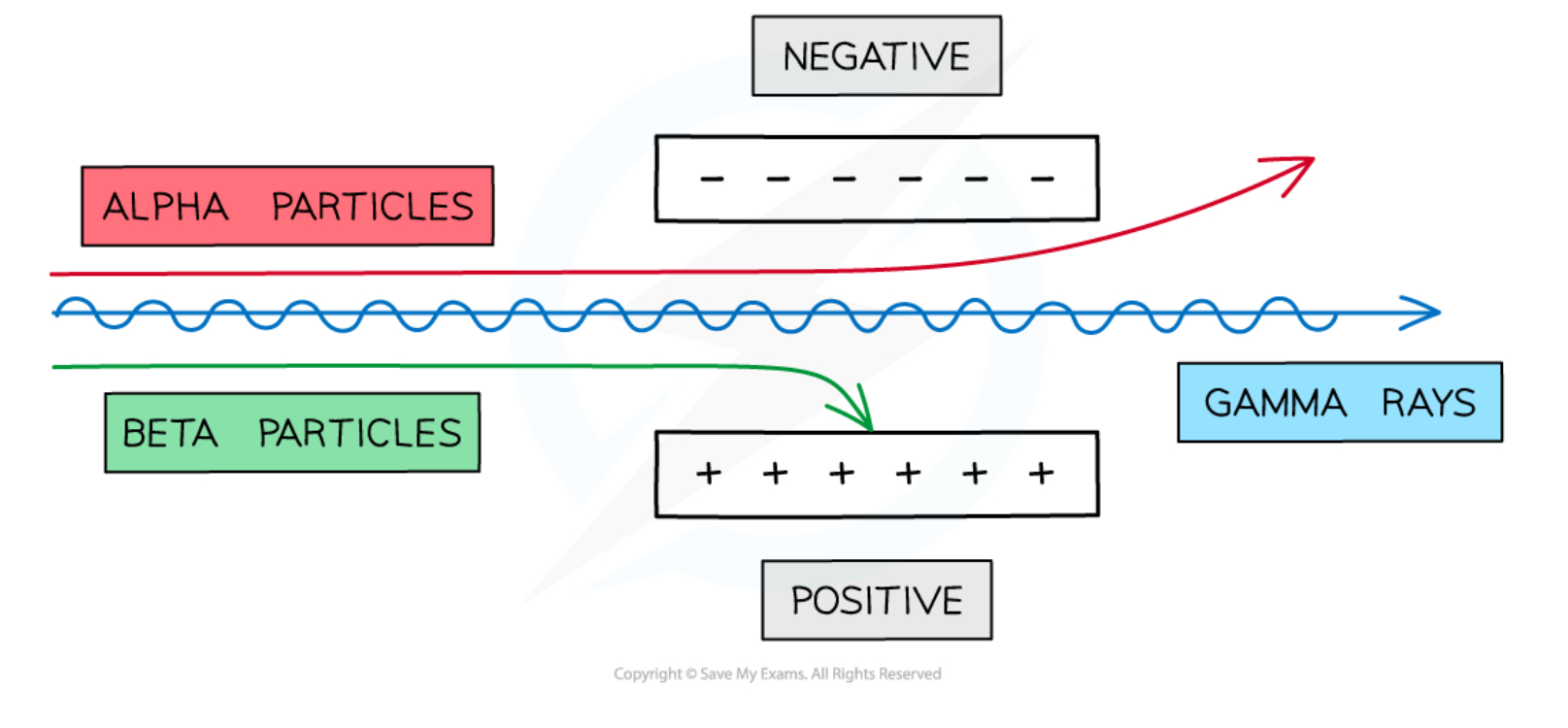
Deflection of α, β−, γ particles in an electric field
α → deflected towards the negative plate
β− → deflected towards the positive plate
γ → not deflected and travels straight through between the plates
Deflection of α, β−, γ particles in a magnetic field
Alpha particles can be deflected slightly in strong electric and magnetic fields
Alpha particles have the highest charge, but also the greatest mass, so their high momentum means they deflect less than a beta particle (in a given field)
Beta particles can be deflected through large angles by electric and magnetic fields
Beta particles typically travel at much greater speeds than alpha particles, but have much less mass, so they deflect significantly more than an alpha particle (in a given field)
Gamma rays are not deflected in magnetic and electric fields as they are electrically neutral
However, they can transfer their energy to atomic electrons which can be deflected
Applications of α & β−
α → smoke detectors
β− → thickness control
Safe handling of radioactive sources
- Keeping radioactive sources shielded when not in use, for example in a lead-lined box
- Wearing protective clothing to prevent the body from becoming contaminated
- Keeping personal items outside of the room to prevent them from becoming contaminated
- Limiting exposure time so less time is spent with radioactive materials
- Handling radioactive materials with long tongs to increase the distance from them
- Monitoring the exposure of workers, such as radiographers, using detector badges
Ionising radiation
Radiation that produces ions in the substances it passes through. It destroys cell membranes and damages vital molecules such as DNA directly or indirectly by creating 'free radical' ions which react with vital molecules
Penetrating power
Alpha particles can be stopped by a single sheet of paper
Beta particles can be stopped by a few millimetres of aluminium foil
The intensity of gamma radiation can be reduced by several metres of concrete or several centimetres of lead
Count rate
Number of counts (e.g. recorded by a Geiger tube) per unit of time
Intensity (of radiation)
Radiation energy per unit time passing normally through unit area
Background radiation
A measure of the level of ionising radiation present in the environment at a particular location
- can be natural or artificial
Natural sources of background radiation
50% - Radon gas: comes from rocks, stone & brick due to the presence of radioactive elements like uranium // very low ∴ not a health concern
11% - Radioactive material in food & drink
10% - Cosmic rays
Artificial sources of background radiation
13% - Nuclear medicine: X-rays, CT scans, radioactive tracers etc
Other 1% - Nuclear waste, Nuclear fallout from nuclear weapons, Nuclear accidents
Radioactive decay
A RANDOM and SPONTANEOUS process where the nucleus breaks down into a more STABLE nucleus through the emission of alpha, beta or gamma
Random in terms of radioactive decay
Can't predict which or when a decay will happen
Spontaneous in terms of radioactive decay
Without external influences
Stable in terms of radioactive decay
Lower energy level
Decay curve
An exponential decrease curve showing how the mass or activity of a radioactive isotope decreases with time
Half-life
The half-life, T1/2, of a radioactive isotope is the time taken for the mass of the isotope to decrease to half the initial mass
Activity
The activity of a radioactive isotope is the number of nuclei of the isotope that disintegrate per second.
Measured in Becquerel (Bq) where 1 Bq = 1 disintegration per second
Decay constant, λ
The probability of an individual nucleus decaying per unit of time
Radioactive decay equation HELPP
- the greater the decay constant, the greater the activity of the sample
- the activity depends on the number of undecayed nuclei remaining in the sample
- the minus sign indicates that the number of nuclei remaining decreases with time
MODELLING W/ CONSTANT DECAY PROBABILIT
Half-life equation
T₁/₂ = ln2/λ
k = wavelength
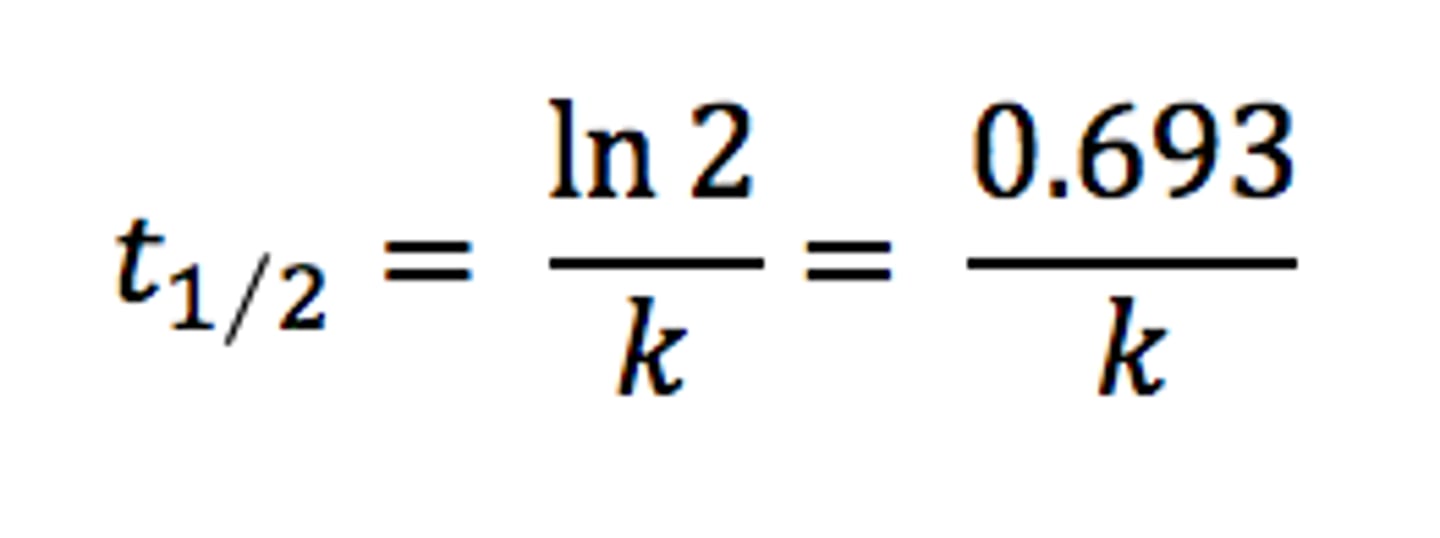
Use of activity equation
A = λN
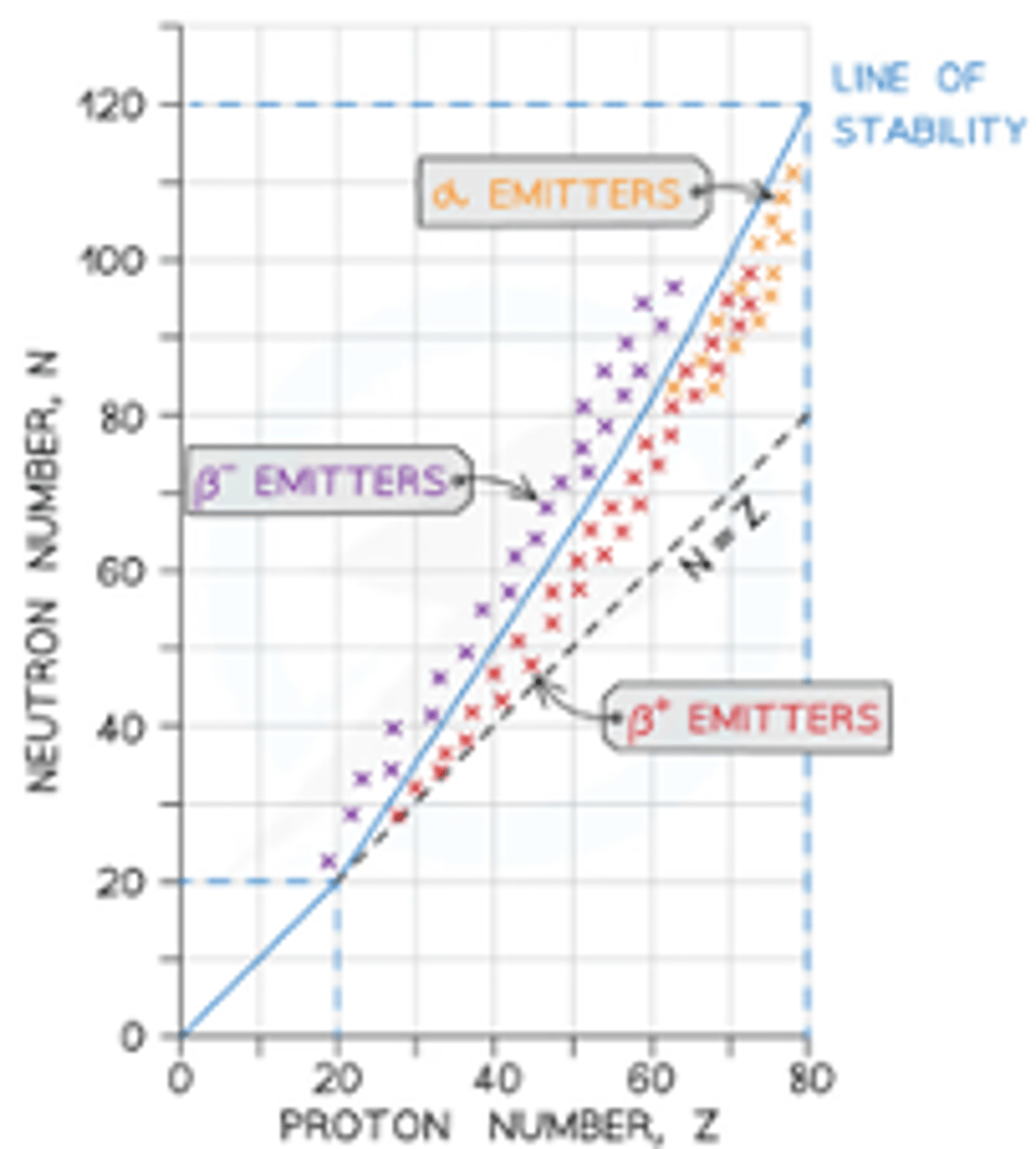
Graph of N against Z for stable nuclei
What makes a nucleus unstable
Too many neutrons
Too many protons
Too many nucleons ie. too heavy
Too much energy
Importance of neutron-proton ratio in nuclei stability
- At a short range (around 1-3 fm), nucleons are bound by the strong nuclear force
- Below 1 fm, the strong nuclear force is repulsive in order to prevent the nucleus from collapsing
- At longer ranges, the electromagnetic force acts between protons, so more protons cause more instability
- Therefore, as more protons are added to the nucleus, more neutrons are needed to add distance between protons to reduce the electrostatic repulsion
Also, the extra neutrons increase the amount of binding force which helps to bind the nucleons together
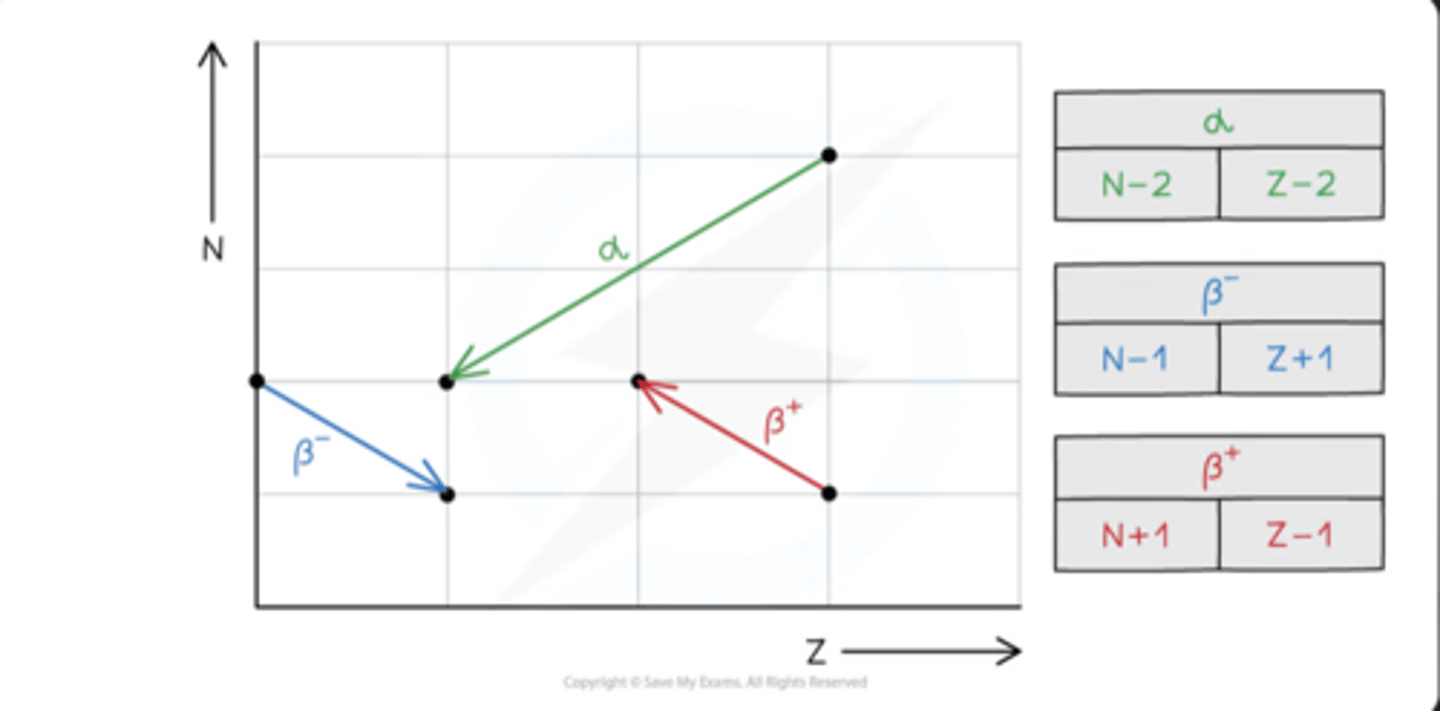
Isotope decay - alpha emission
- emitters found beneath the line of stability when Z > 82 where there are too many nucleons in the nucleus
- nuclei have more protons than neutrons, but they are too large to be stable due to strong nuclear force between the nucleons is unable to overcome the electrostatic force of repulsion between the protons
Isotope decay - beta-minus emission
- emitters found to the left of the stability line where the isotopes are neutron-rich compared to stable isotopes
- a neutron is converted to a proton and emits a β⁻ particle (and an anti-electron neutrino)
Isotope decay - beta-plus emission
- emitters found to the right of the stability line where the isotopes are proton-rich compared to stable isotopes
- A proton is converted into a neutron and emits a β⁺ particle (and an electron neutrino)
Isotope decay - electron capture
- emitters found on the right of the stability line where the isotopes are proton-rich compared to stable isotopes
- electron capture occurs when a nucleus captures one of its own orbiting electrons
- similar to β⁺ decay, a proton in the nucleus is converted into a neutron, releasing a gamma-ray (and an electron neutrino)
What happens when there's too many neutrons
BETA-MINUS EMISSION OCCURS
- neutron in nucleus changes into proton, releasing a β⁻ particle (an electron) and an anti-electron neutrino
- number of neutrons decreases by one
- number of protons increases by one
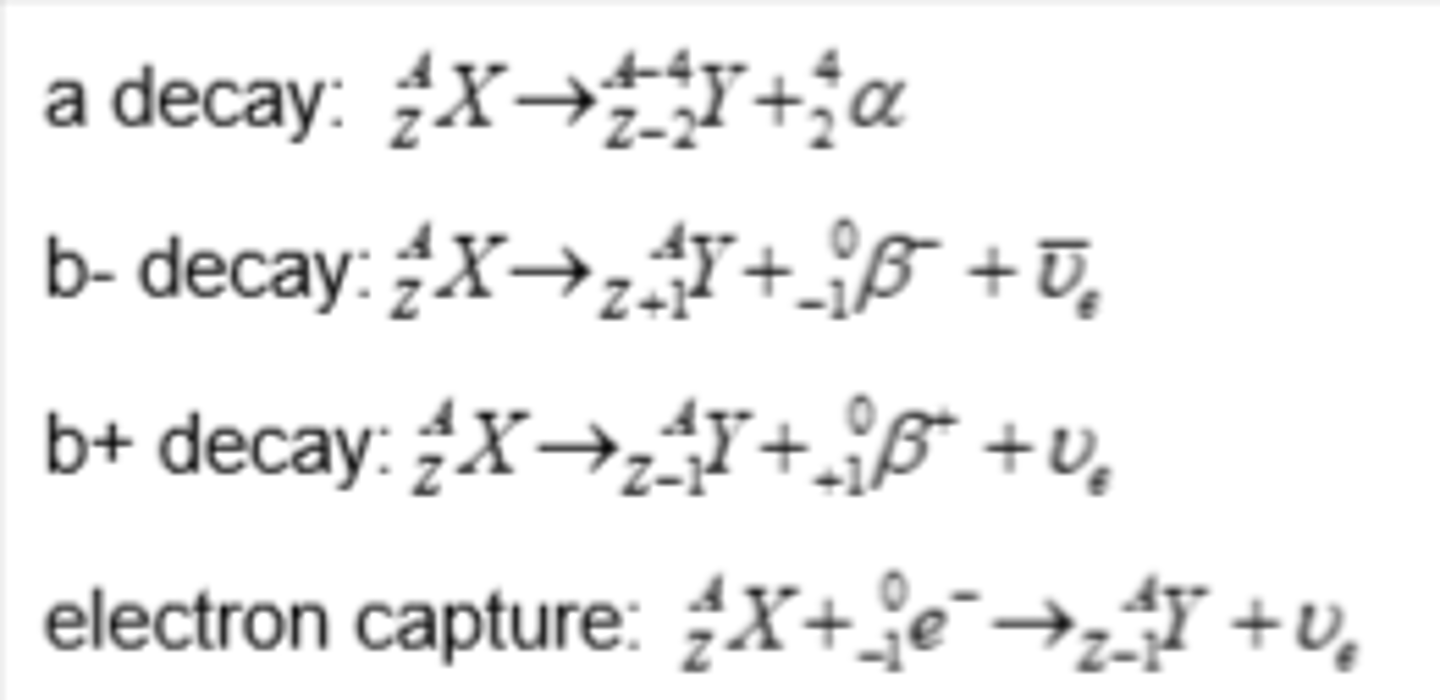
What happens when there's too many proton
BETA-PLUS EMISSION or ELECTRON CAPTURE
- BETA-PLUS: proton changes into a neutron emits a β⁺ particle (a positron) and an electron neutrino
- ELECTRON CAPTURE: an orbiting electron is taken in by the nucleus and combined with a proton causing the formation of a neutron and neutrino
Both cause:
- the neutron number increases by 1
- the proton number decreases by 1
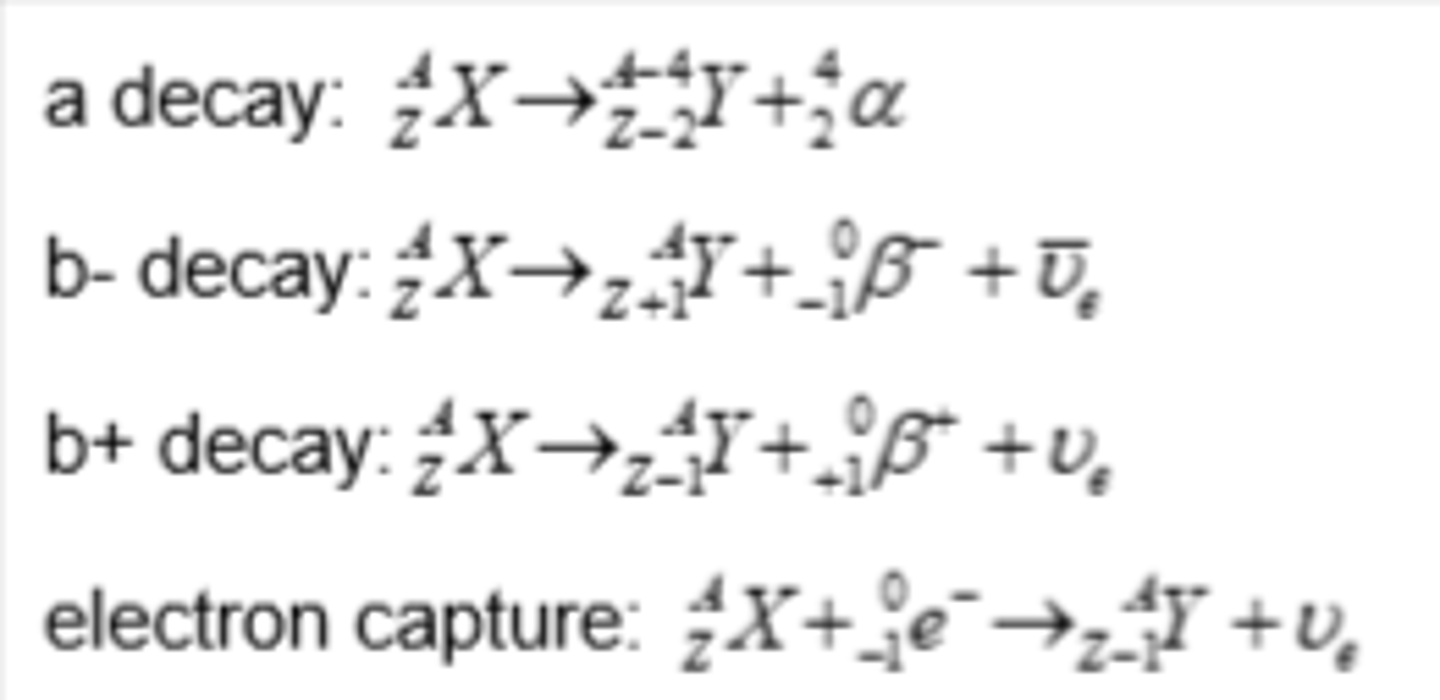
What happens when there's too many nucleons
ALPHA EMISSION
- helium nuclei
- neutron number decreases by 2
- proton number decreases by 2
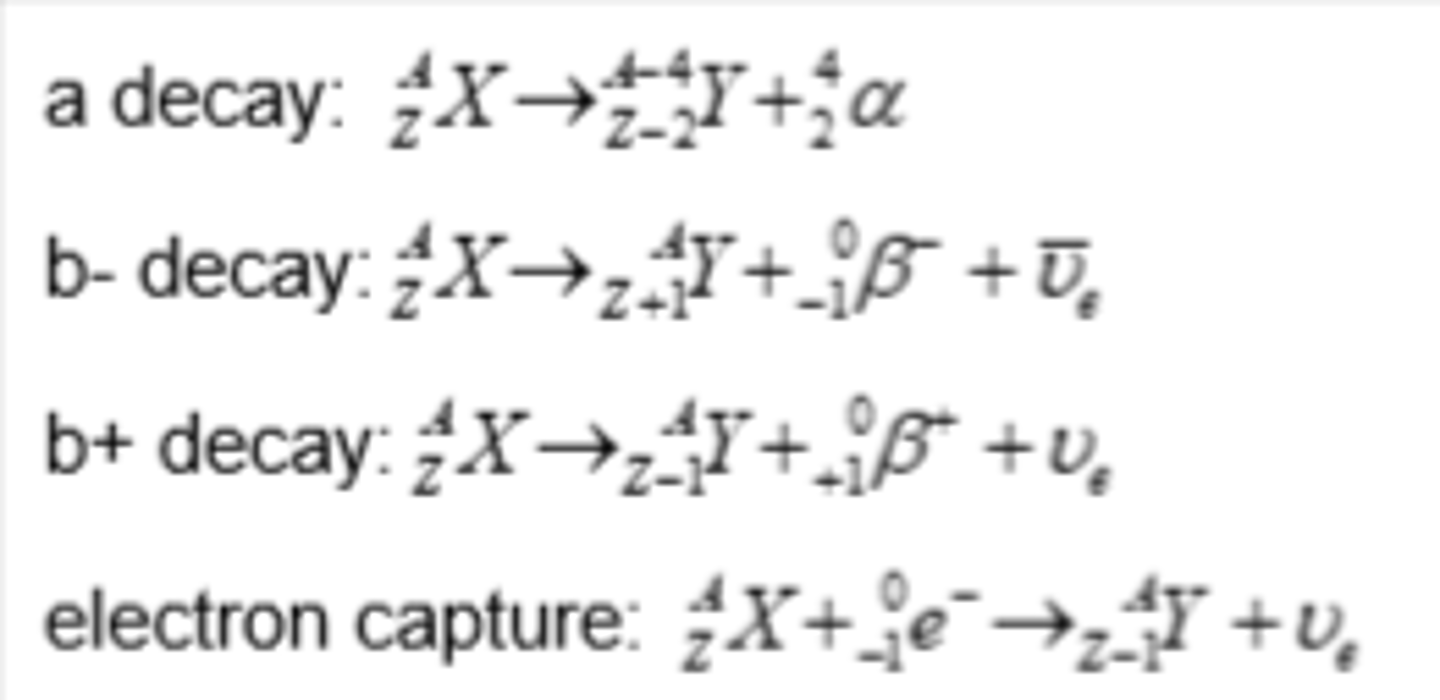
What happens when there's too much energy
GAMMA EMISSION OCCURS
- high-energy electromagnetic radiation
- usually occurs after a different type of decay, such as alpha or beta decay due to the nucleus becomes excited and has excess energy
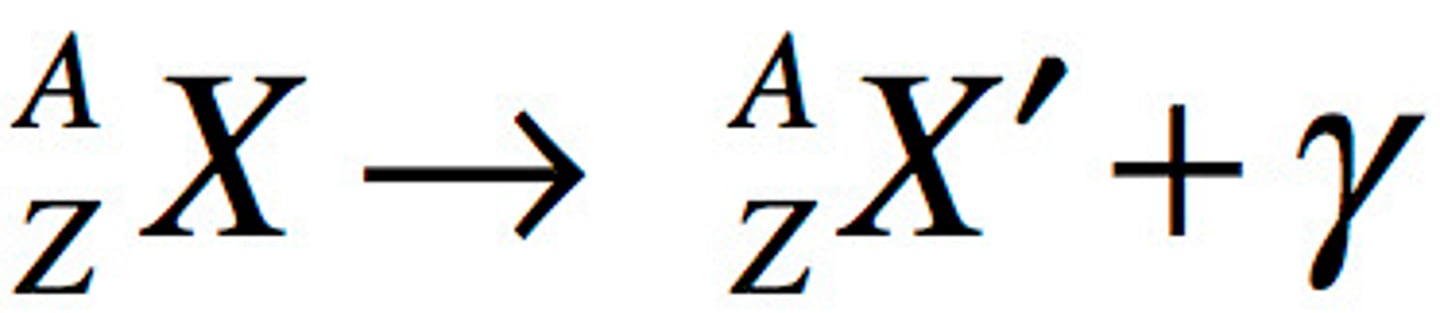
Representing Nuclear processes graphically
Nuclear ground state
The lowest energy state of a nucleus
Nuclear excited state
A nucleus which is an energy state higher than its ground state
Nuclear energy level diagram
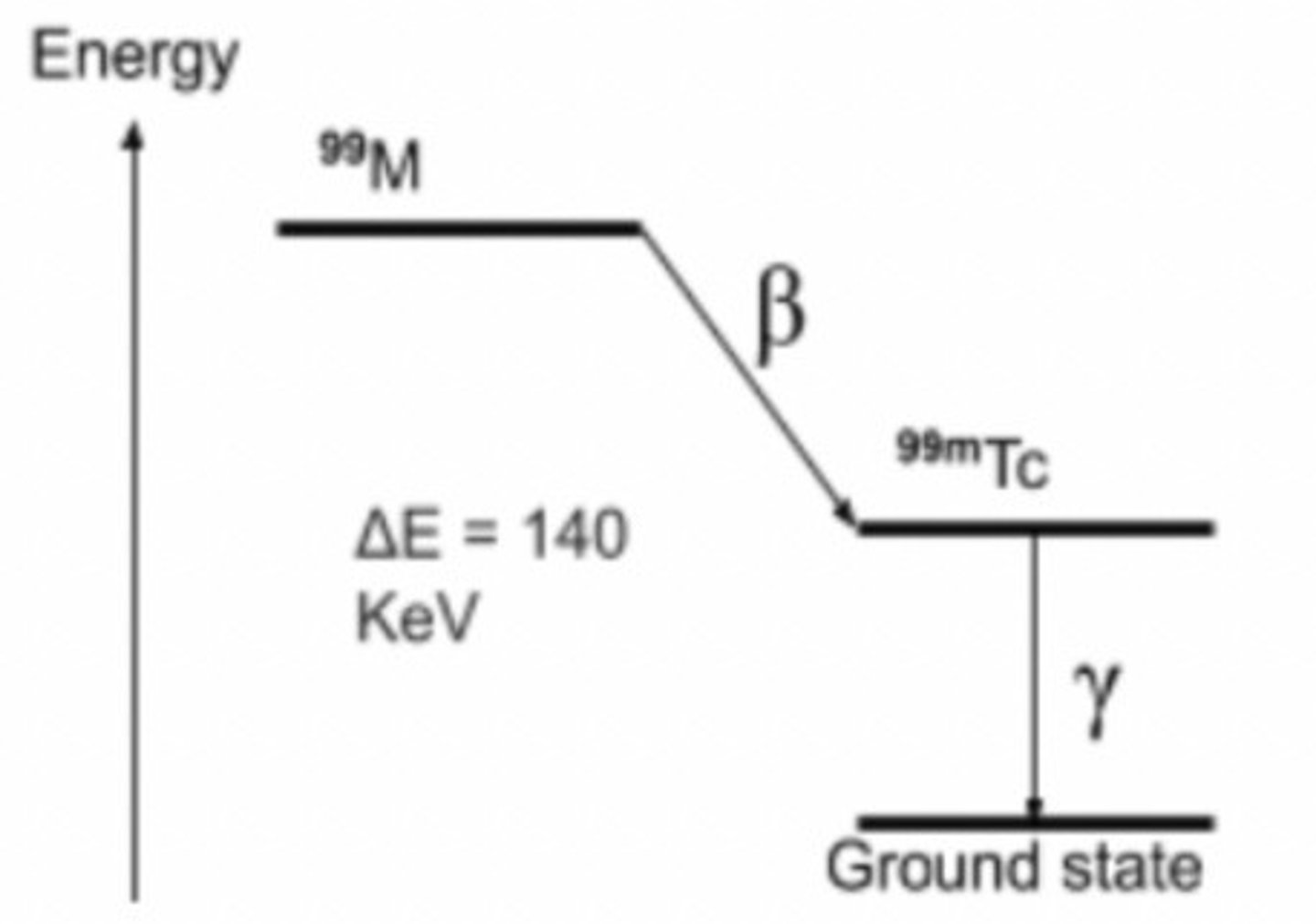
Nuclear metastable state
An excited state of the nuclei of an isotope that lasts long enough after α or β emission for the isotope to be separated from the parent isotope
- referred to as 'm'
Applications of technetium-99m
⁹⁹ᵐTc is a product of molybdenum-99 (a product in nuclear reactors)
- ⁹⁹Mo has a half life of 66 hours, therefore long enough to be transported to hospitals
- ⁹⁹ᵐTc has a short half0life of 6 hours
- this is an adequate timeframe for examining a patient and is short enough to minimise damage to the patient
Binding energy
The work that must be done to separate a nucleus into its constituent neutrons and protons
Binding energy per nucleon
The average work done per nucleon to separate a nucleus into its constituent neutrons and protons
Mass defect
The mass defect of a nucleus is the difference between the mass of the separated nucleons and the nucleus
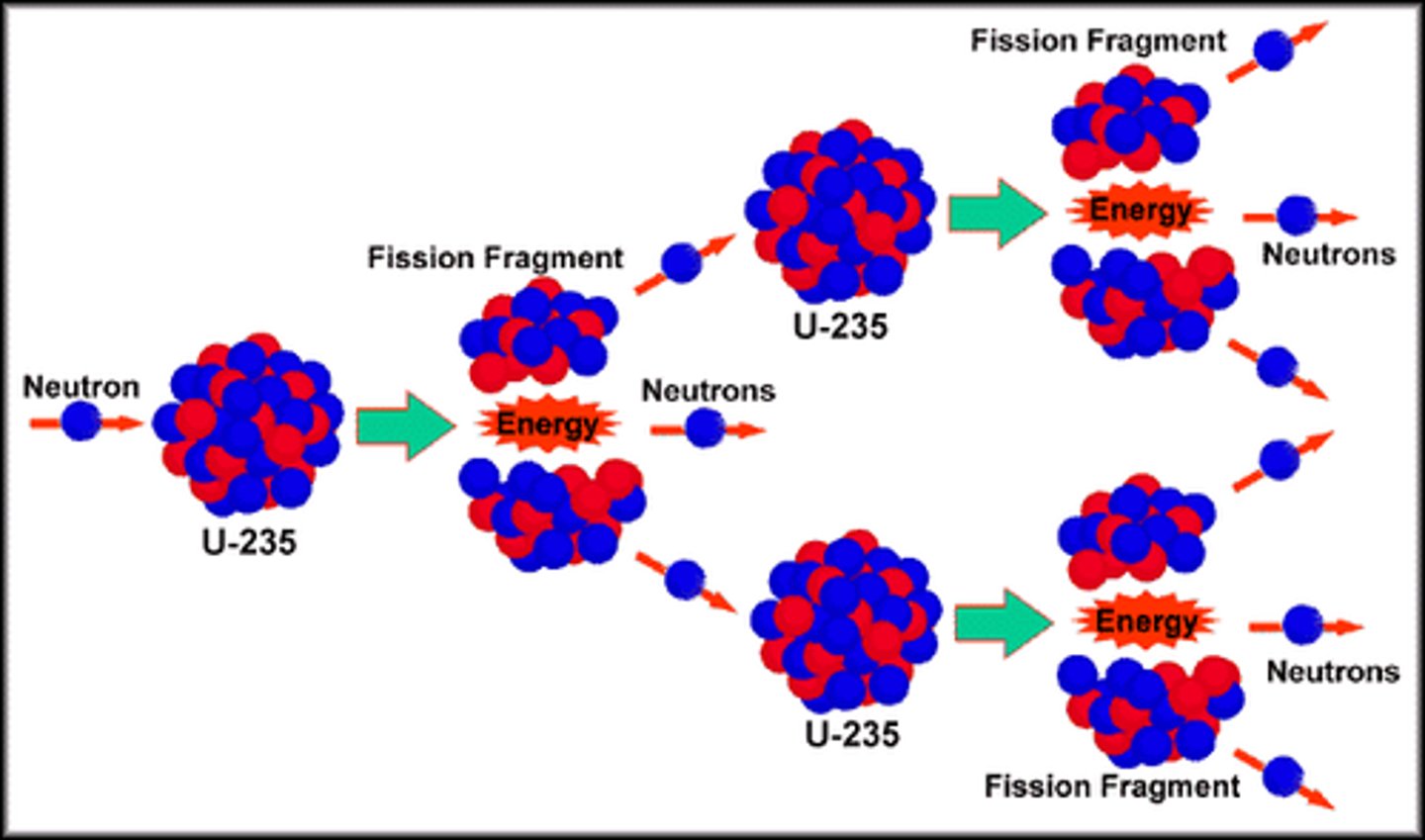
Nuclear fission
The splitting of a large nucleus (e.g. of uranium-235) into two approximately equal
fragments. Induced fission is fission caused by an incoming neutron colliding with the nucleus.
Nuclear fusion
The fusing together of light nuclei to form a heavier nucleus
Nuclear fusion process
- low mass nuclei can undergo fusion & release energy (e.g. hydrogen & helium)
- when two protons fuse, the element deuterium is produced
- in the centre of stars, the deuterium combines with a tritium nucleus to form a helium nucleus (also releases energy which provides fuel for starts to continue burning)
- for two nuclei to fuse, both must have the same kinetic energy
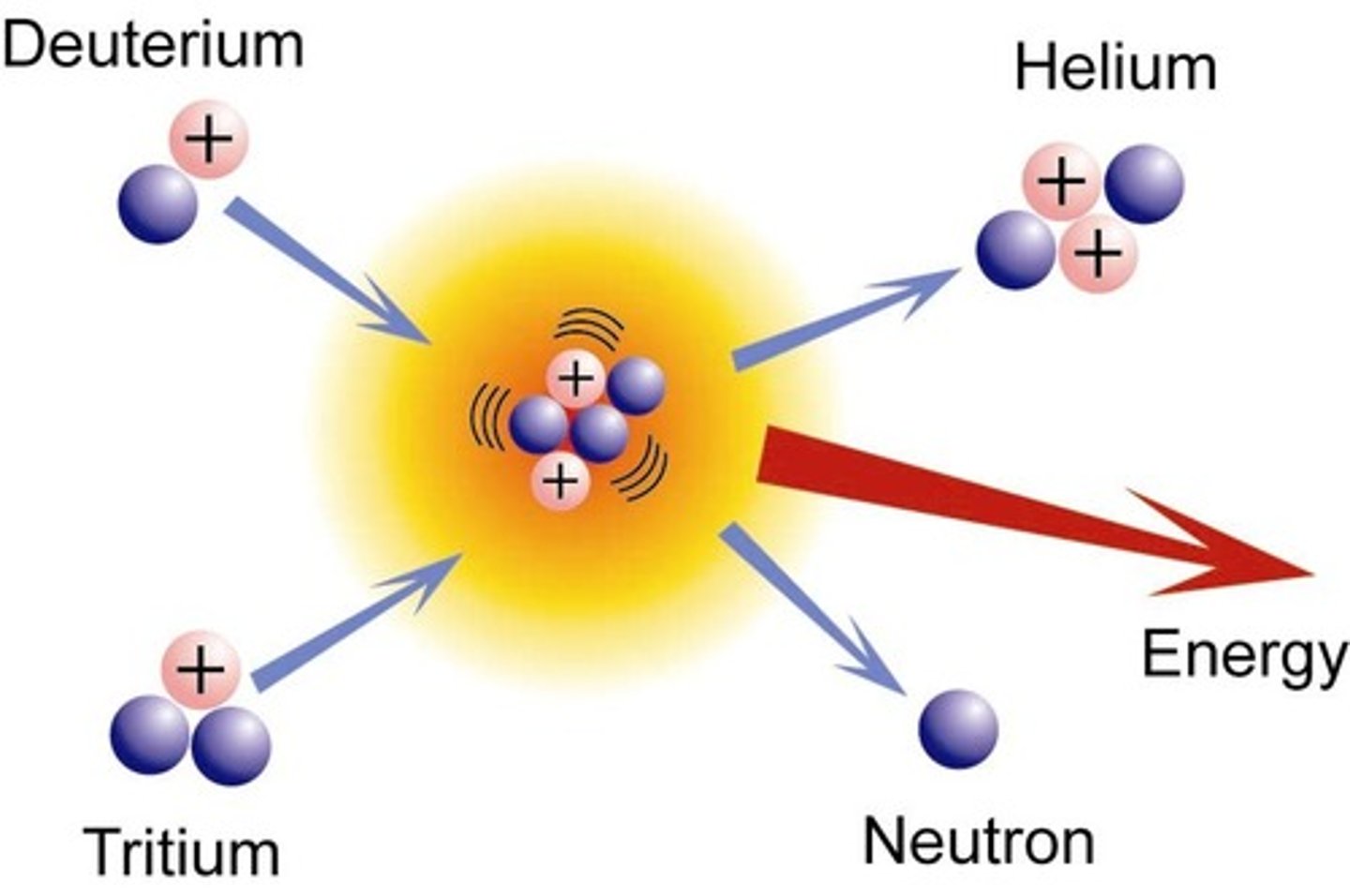
Why does fusion only occur in extremely hot environments e.g. core of a star
Takes a lot of energy to overcome the electrostatic force
Fission neutrons
Neutrons released when a nucleus undergoes fission.
Fission neutrons process
MUST BE INDUCED
- done by firing thermal neutrons at the nuclei
- the nucleus absorbs/captures the thermal neutron making it more unstable
- this causes it to spit into smaller nuclei
- neutrons are ejected from the nucleus
- these can collide with other nuclei triggering a cascade effect
- leads to a chain reaction which lasts until all materials have undergone fission or the reaction is halted
Key features of a nuclear reactor
1) fuel roads
2) control rods
3) moderator
44) coolant
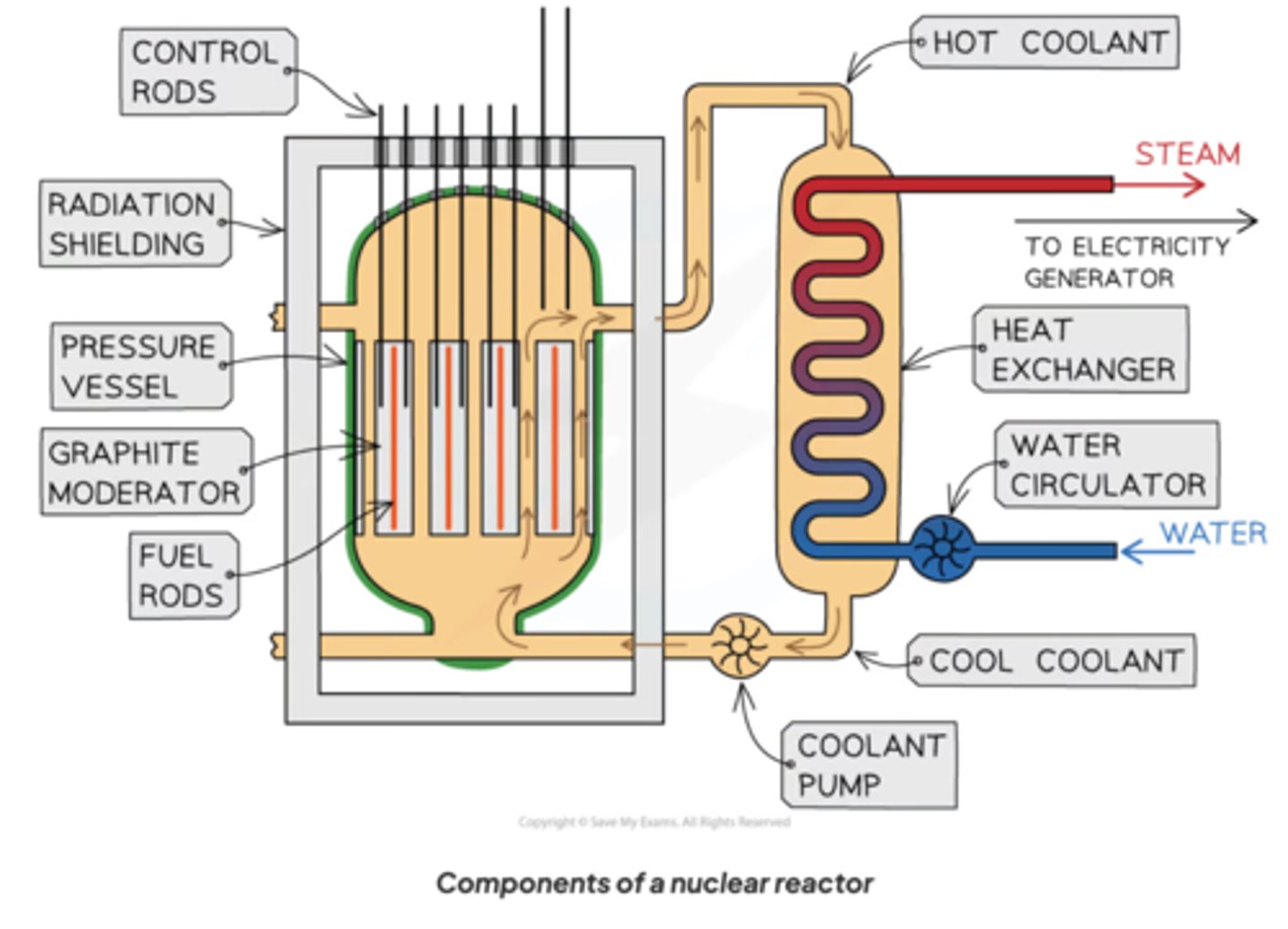
Reactor core
The fuel rods and the control rods together with the moderator substance are in a steel vessel through which the coolant (which is also the moderator in a pressurised water reactor) is pumped
Fuel rods
Contains fuel, e.g. enriched uranium
The fuel is fed to the reactor inside the fuel rods in canisters of stainless steel
Control rods
Made of a neutron-absorbing substance such as cadmium or boron, the controls rods are moved in or out of the core of a nuclear reactor to control the rate of fission events in the reactor.
Moderator
The moderator substance in a thermal nuclear reactor slows the fission neutrons down so they can go on to cause further fission.
- materials like water & graphite
- a thermal nuclear reactor is simply a nuclear reactor that has a moderator in the core
Coolant
Water is good coolant as it has a high SHC
The purpose is to absorb & transfer the heat released by the fission reaction
Critical mass
The minimum mass of a fissile isotope in a nuclear reactor necessary to produce a chain reaction
Sub-critical mass
Less than the critical mass
- reaction eventually stops
Super critical mass
More mass than the critical mass
- could lead to the reaction becoming uncontrollable)
Radius of atom & nucleus
Atom: 1 x 10⁻¹⁰ m
Nucleus: 1 x 10⁻¹⁴ m
atom is 10,000 times bigger
Measuring nuclear radius - closest approach method
- shoot α particles at a gold nucleus
- as the α particle approaches the gold, its Ek↓ & Ep↑
- equate ΔW = electric potential to get potential energy
- equate potential energy to Ek (like picture)
- calculate radius
- repulsion prevents the α from getting any closer
REMEMBER THIS IS MAXIMUM POTENTIAL ENERGY
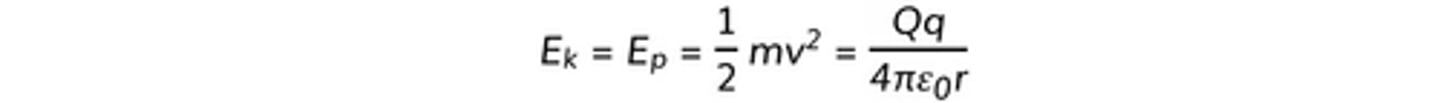
Measuring nuclear radius - closest approach method advantages
1. Alpha scattering gives a good estimate of the upper limit for a nuclear radius
2. The mathematics behind this approach are very simple
3. The alpha particles are scattered only by the protons and not all the nucleons that make up the nucleus
Measuring nuclear radius - closest approach method disadvantages
1. Alpha scattering does not give an accurate value for nuclear radius as it will always be an overestimate
2. Alpha particles contain hadrons which are affected by the strong nuclear force but the mathematics in this method cannot account for the effects caused by the force
3. The gold nucleus will recoil as the alpha particle approaches
4. Very few alpha particles rebound at exactly 180°, so to detect these, a small collision region is required
5. The alpha particles in the beam are assumed to have the same initial kinetic energy which may not be realistic
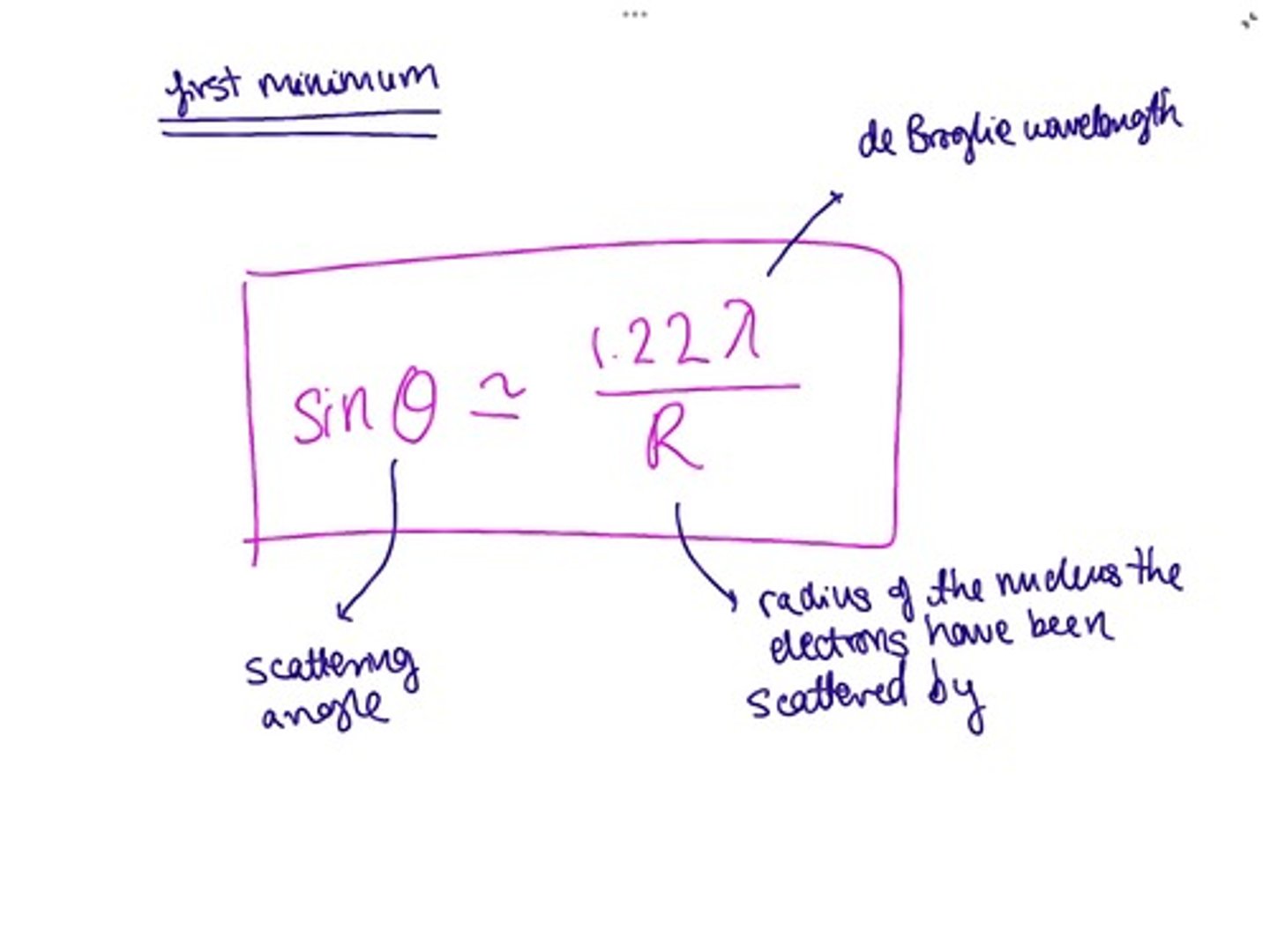
Measuring nuclear radius - electron scattering
- electrons are accelerated towards a thin gold sheet by a very high pd giving them a high speed
- they diffract giving a diffraction pattern
- obtain circular fringes, bright central circular fringe followed by concentric circles of bright & dark fringes
- to calculate θ measure distance between centre to first minima (n=1)
put into equation
Measuring nuclear radius - electron scattering advantages
1. Electron scattering is much more accurate than the closest approach method
2. This method gives a direct measurement of the radius of a nucleus
3. Electrons are leptons, so they are not affected by the strong nuclear force
Measuring nuclear radius - electron scattering disadvantages
1. Electrons must be accelerated to very high speeds to maximise the resolution
- significant diffraction takes place when the electron wavelength is similar to the size of the nucleus
- higher speeds are needed to shorten the de Broglie wavelength
2. Electrons can be scattered by both protons and neutrons
- if there is an excessive amount of scattering, then the first minimum of the electron diffraction can be difficult to determine
Appreciate that there's a balance between risk and benefits in the use of radiation in medicine
Okay?
Radiocarbon dating
(equation + relability)
¹₁H = proton
Samples of living material can be tested by comparing the current amount of carbon-14 in them and compared to the initial amount (which is based on the current ratio of carbon-14 to carbon-12), and hence they can be dated
- highly reliable method for estimating the ages of samples between 500 and 60 000 years old
Uranium-Lead dating
- Initially, there is only uranium in the rock, but over time, the uranium decays via a decay chain which ends with lead-206, which is a stable isotope
- Uranium-238 has a half-life of 4.5 billion years
- Over time, the ratio of lead-206 atoms to uranium-238 atoms increases
- The ratio of uranium to lead in a sample of rock can then be used to determine its age
Applications of radioactivity
Main uses:
- Nuclear power
- In medicine e.g. radiotherapy, tracers and sterilising equipment
- Radiocarbon dating of archaeological artefacts
- Uranium-lead dating of rock samples
- Radioisotope power systems
Equations for radioactive decay
N = number of decayed nuclei at a certain time
A = activity at a certain time

Nuclear radius, R against nucleon number, A
(description and what it means)
- The graph starts with a steep gradient at the origin
- Then the gradient gradually decreases to almost horizontal
- As more nucleons are added to a nucleus, the nucleus gets bigger
- However, the number of nucleons A is not proportional to its size r

Nuclear Radius Equation
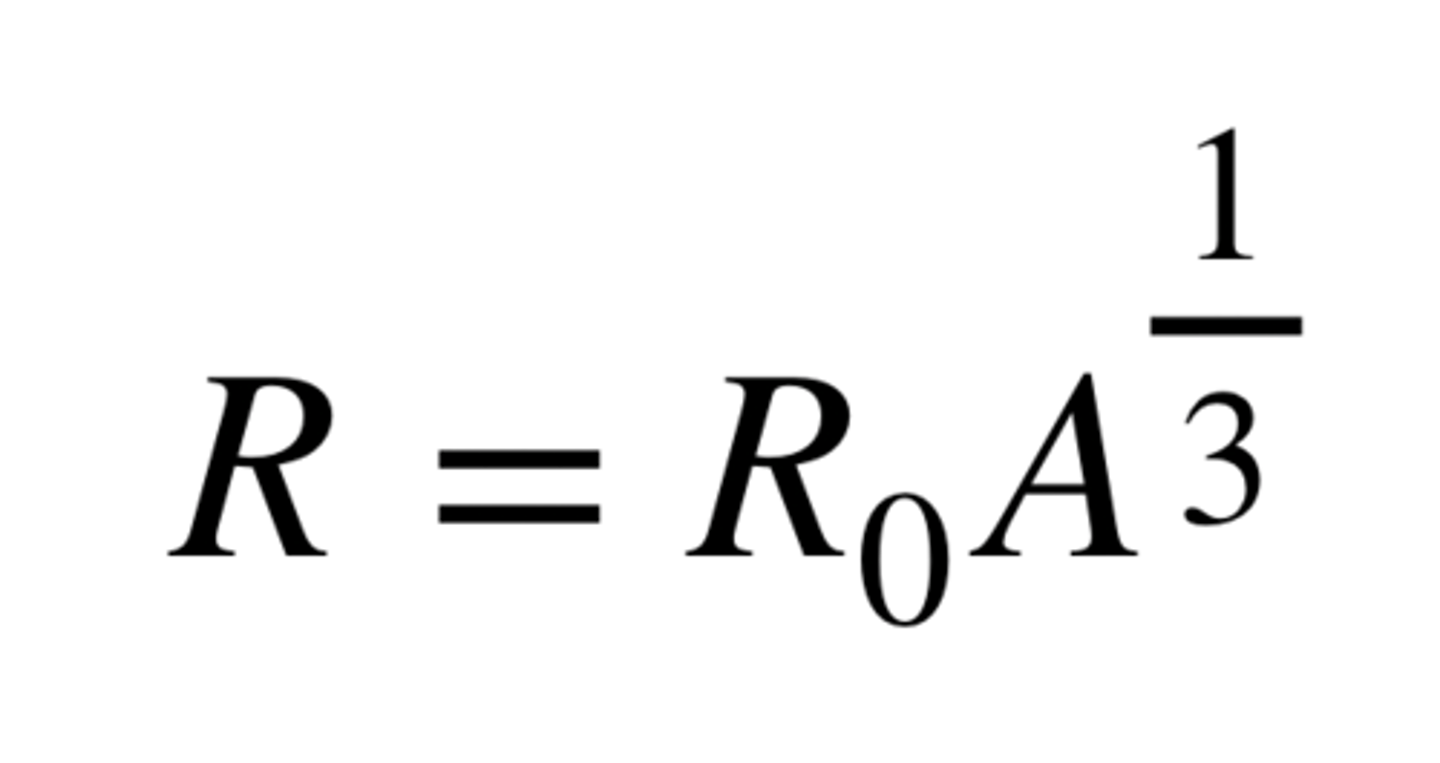
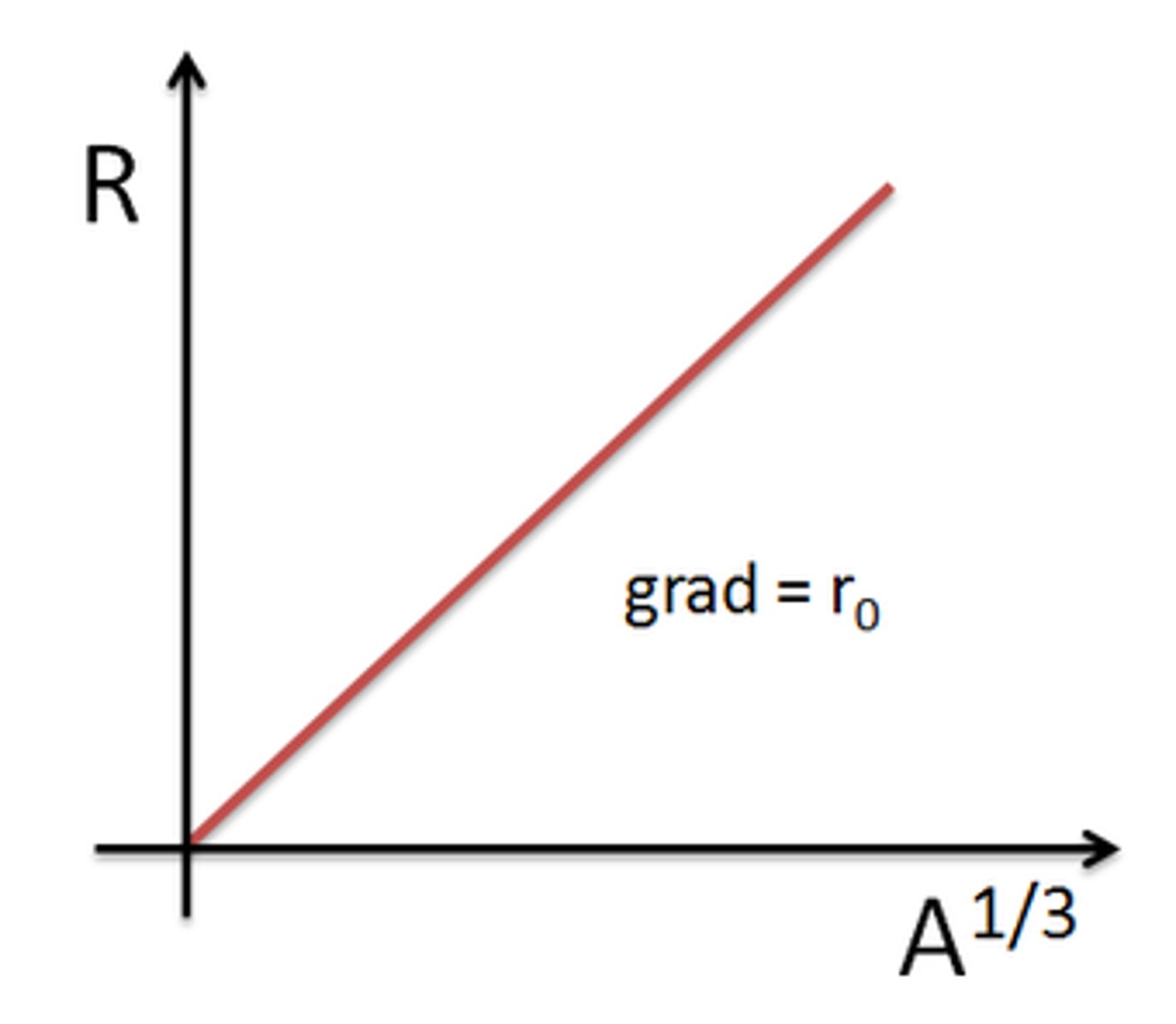
Graph of R against A⅓
Straight line through the origin with the gradient equal to R₀
Calculating density of nuclear material equation
The fact that nuclear density is constant shows that nucleons are evenly separated throughout the nucleus regardless of their size

What does nuclear density being significantly larger than atomic density mean
The majority of the atom's mass is contained in the nucleus
The nucleus is very small compared to the atom
Atoms must be predominantly empty space
Intensity against angle of electron diffraction graph

Mass Difference & Binding Energy ADD
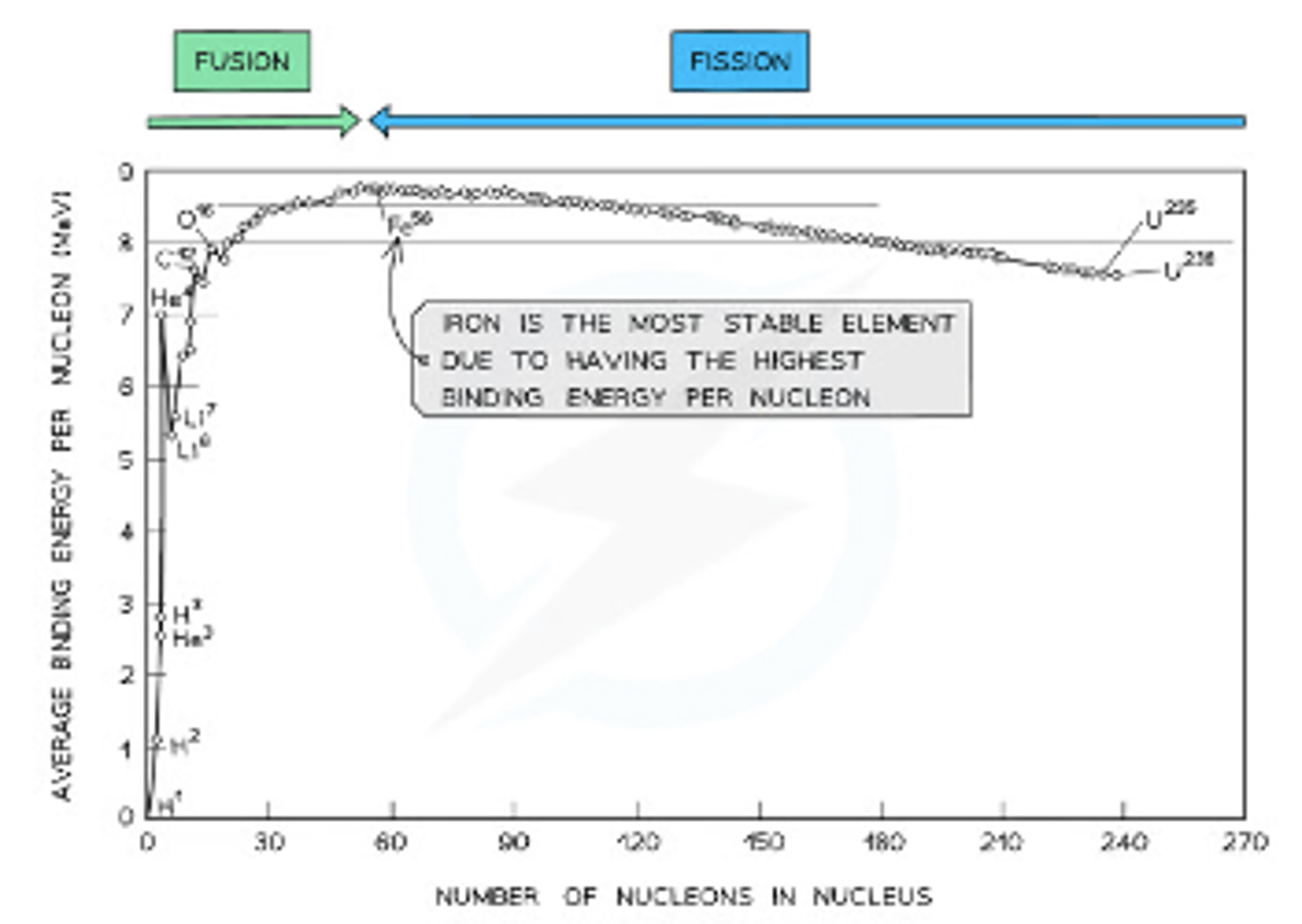
Graph of average binding energy per nucleon against nucleon number
Low values of :
-
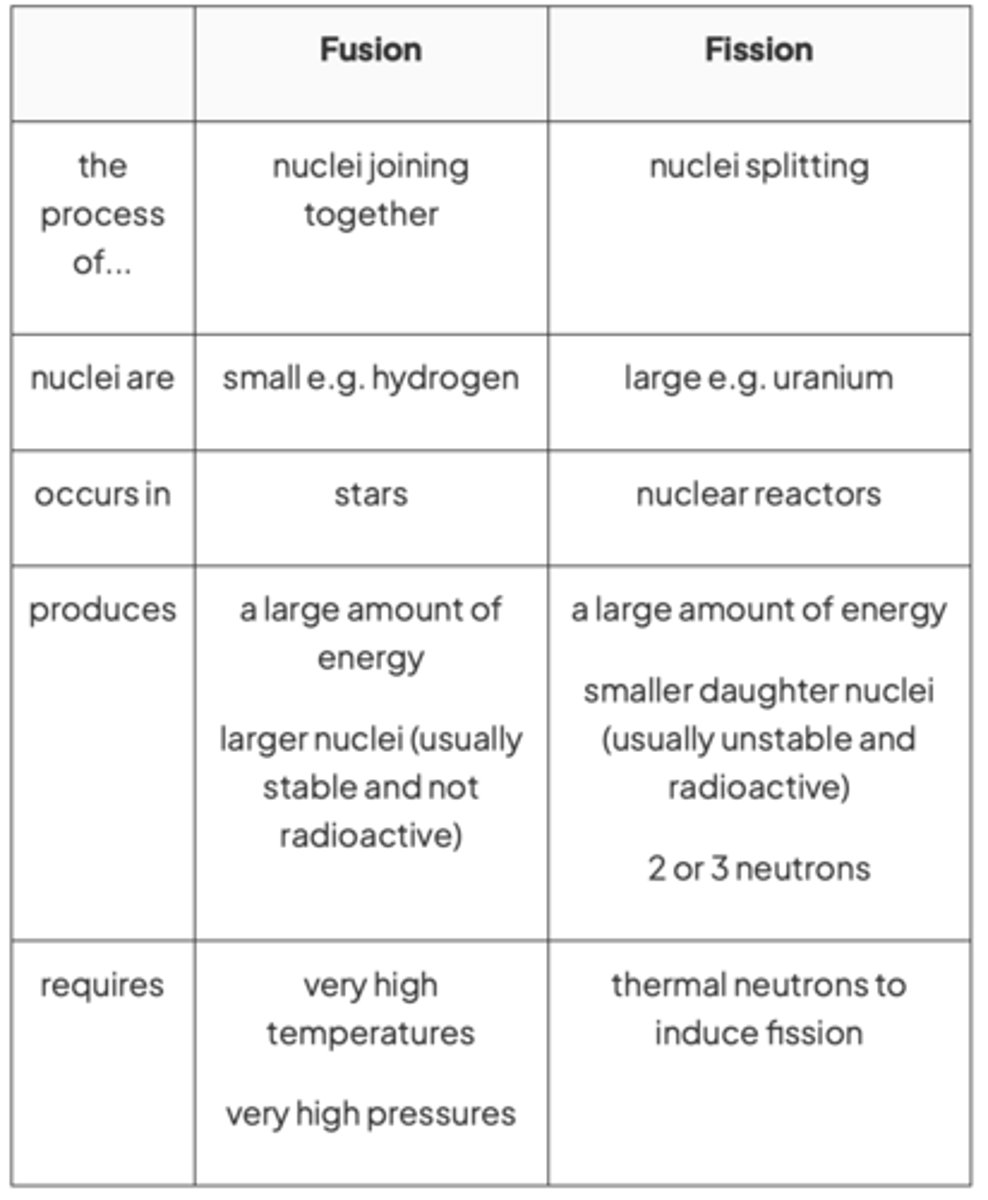
Similarities between fusion & fission
Both fusion and fission reactions release energy
Differences between fusion & fission
Induced fission
A stable nucleus splits into small nuclei due to the absorption of a slow-moving neutron
High level waste
Most dangerous, remain radioactive for thousands of years
- extremely hot fuel rods that require additional care when being handled & stored
Low level waste
Waste such as clothing, gloves & tools which may be lightly contaminated
- will be radioactive for a couple of years so must be encased in concrete & a few metres underground until it can be disposed with regular waste
Nuclear waste treatment
- non-renewable energy resources
- production of radioactive waste is very dangerous and expensive to deal with, with long-lasting effects
- commissioning and decommissioning a nuclear power station is expensive and time-consuming
- a nuclear meltdown, could have catastrophic consequences on the environment and on the people living in the surrounding area