Molecular forensic science
1/84
Earn XP
Description and Tags
1-3 chapters
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
85 Terms
An astronaut uses a laboratory balance and weighs an object on earth and again on the moon. Which statement below about the weight and mass of the object is true?
The mass will be the same on earth and moon but the weight will be less on the moon.
Most of the alpha particles directed at a thin gold foil in Rutherford's experiment
passed directly through the foil undeflected.
Which of the following represent isotopes?
A and C
Which are isotopes? An atom that has an atomic number of 34 and a mass number of 76 is an isotope of an atom that has
an atomic number of 34 and a mass number of 80.
Which of the following two atoms are isotopes?
12C6 and 13C6
passed directly through the foil undeflected.
an electron
The existence of neutrons in the nucleus of an atom was demonstrated by
None of the above
Which of the following is not explained by Dalton's atomic theory?
the existence of more than one isotope of an element
The current model of the atom in which essentially all of an atom's mass is contained in a very small nucleus, whereas most of an atom's volume is due to the space in which the atom's electrons move was established by
Rutherford's gold foil experiment
The charge-to-mass ratio of an electron was established by
Thomson's cathode ray tube experiment
The existence of electrons in atoms of all elements was demonstrated by
Thomson's cathode ray tube experiment
Elements A and Q form two compounds. The ratio (mass Q)/(mass A) for compound one is 0.271 and ratio (mass Q)/(mass A) for compound two is 0.362. If compound one has the chemical formula AQ, what is the chemical formula for compound two?
A3Q4
Elements A and Q form two compounds, AQ and A2Q. Which of the following must be true?
(mass Q)/(mass A) for AQ must be 2 times (mass Q)/(mass A) for A2Q
Elements A and Q form two compounds, AQ and A2Q3. The mass ratio (mass Q)/(mass A) for AQ is 0.574. What is the mass ratio (mass Q)/(mass A) for A2Q3?
0.861
Which of the following is a part of Dalton's atomic theory?
Atoms are rearranged but not changed during a chemical reaction.
Which of the following statements is not a postulate of Dalton's atomic theory
Atoms are composed of protons, neutrons, and electrons.
24.0 g of which element contains the greatest number of atoms?
B
One mole of which element has the smallest mass?
Ni
What is the standard isotope that is used to define the number of atoms in a mole?
12C
The observation that hydrogen and oxygen can react to form two compounds with different chemical and physical properties, one having an O:H mass ratio = 8:1 and the other having an O:H mass ratio = 16:1 is consistent with the law of
multiple proportions
Identify physical properties (more than one)
Color
Hardness
Convert 7,585 µL to mL
7.585 mL
Convert 6.9 inch to cm
17.53
How many significant figures are in each of the numbers below?
1
200
3
1.27×105
4
2005
2
0.0032
Convert 0.00350 to scientific notation.
3.50×10-3
Write the answer to the mathematical problems below with the correct number of significant figures.
(14.3 − 2.3) ÷ 2.00
6.00
A mass of mercury occupies 0.750 L. What volume would an equal mass of ethanol occupy? The density of mercury is

and the density of ethanol is 0.789 g/mL
12.9 L
Calculate the mass of an object given the density of 2.186 g/mL and volume of 24.22 mL. (Report your answer to 2 decimal places.)
52.94 g
Calculate the volume of an object given the density of 1.910 g/mL and mass of 17.08 g. (Report your answer to 2 decimal places.)
8.94 mL
Calculate the density of an object given the mass of 5.38 g and volume of 4.71 mL. (Report your answer to 2 decimal places.)
1.14 g/mL
Specific gravity of a liquid is often defined as the ratio of the density of a substance to the density of water. If the specific gravity of X relative to water is 0.800 and the specific gravity of Y relative to water is 1.50, which of the following statements is false?
If X is a liquid, Y will float on X.
Which set of numbers below represents measurements that have good precision and good accuracy? The true value of the measurement is 4.75
4.79, 4.68, 4.81, 4.83
Write the answer to the mathematical problems below with the correct number of significant figures.
3967 × 0.022 ÷ 9.09
9.6
Write the answer to the mathematical problems below with the correct number of significant figures.
1.45 + 101 − 11.92
91
Convert 3.3×10-3 to decimal
0.0033
Convert 65 gal/day to L/s
0.0028 L/s
Convert 36 mi/hr to m/s
16
Convert 4,180 cm3 to dm3
4.18
Convert 2,116 mL to L
2.116
convert 0.420 g to mg.
420
Identify whether each of the following phrases relates to mass
Measured in grams
Independent of gravity
Measures the amount of matter
Identify chemical properties (more than one).
Inertness
Explosiveness
Identify physical changes
Formation of clouds and rain
Freezing biological samples for storage
Identify the following as chemical changes (more than one)
Toasting a marshmallow
Burning documents
When measuring a solid metal block at constant temperature, which measurement will change in numerical value depending on the location where it is taken?
weight
What is the chemical symbol for tin?
Sn
Which group 1A element is not a metal?
H
Which of the following elements is a good conductor of heat and electricity?
zinc
All of the following elements are nonmetals except
antimony
transition metals
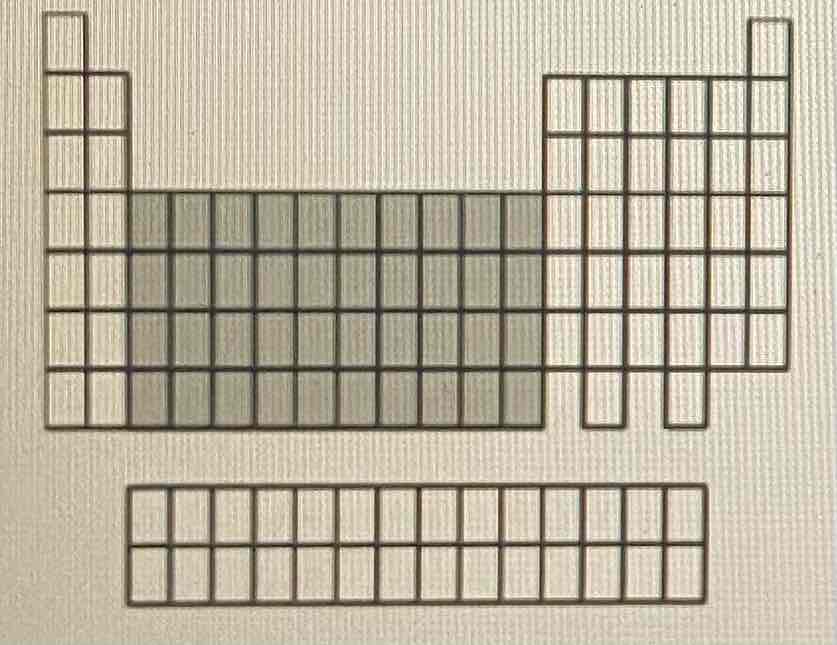
Which of the following statements does not describe a chemical property of oxygen?
The pressure is caused by collision of oxygen molecules with the sides of a container.
Which of the following statements does not describe a physical property of chlorine?
Chlorine combines with sodium to form table salt.
Which horizontal row of the periodic table contains the most elements?
row 6
Which of the following elements has chemical properties similar to oxygen?
Sulfur
Elements in a periodic group have similar
Chemical properties
Most elements in the periodic table are
Metals
Which is not true?
Mendeleev ended each row in his periodic table with an inert gas.
Which element has the chemical symbol, Au?
Gold
Mendeleev arranged the elements according to
atomic weight and chemical reactivity.
Which of the following elements is not a solid at room temperature?
Br
Which group of elements reacts violently with water?
alkali metals
Which of the following elements is a liquid at room temperature?
Mercury
Lithium belongs to the ___group of the periodic table.
alkali metal
The vertical columns of the periodic table are called
Groups
The horizontal rows of the periodic table are called
Periods
Calcium belongs to the What group
alkaline earth
Argon belongs to the what group
noble gas
Bromine belongs to
halogen
Chemically similar to E
B
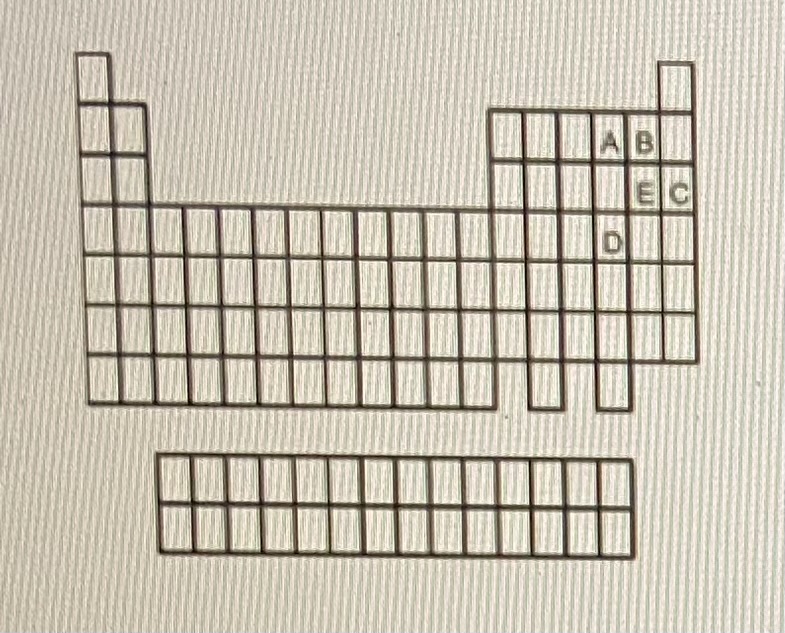
Inner transition metal
Halageon
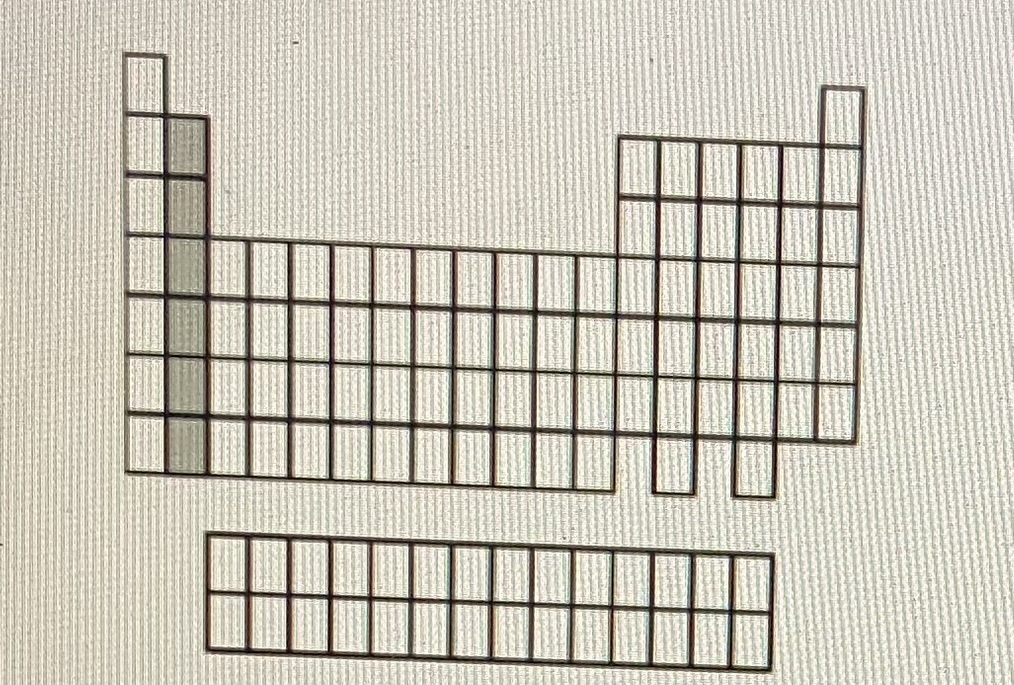
Alkaline earth metals
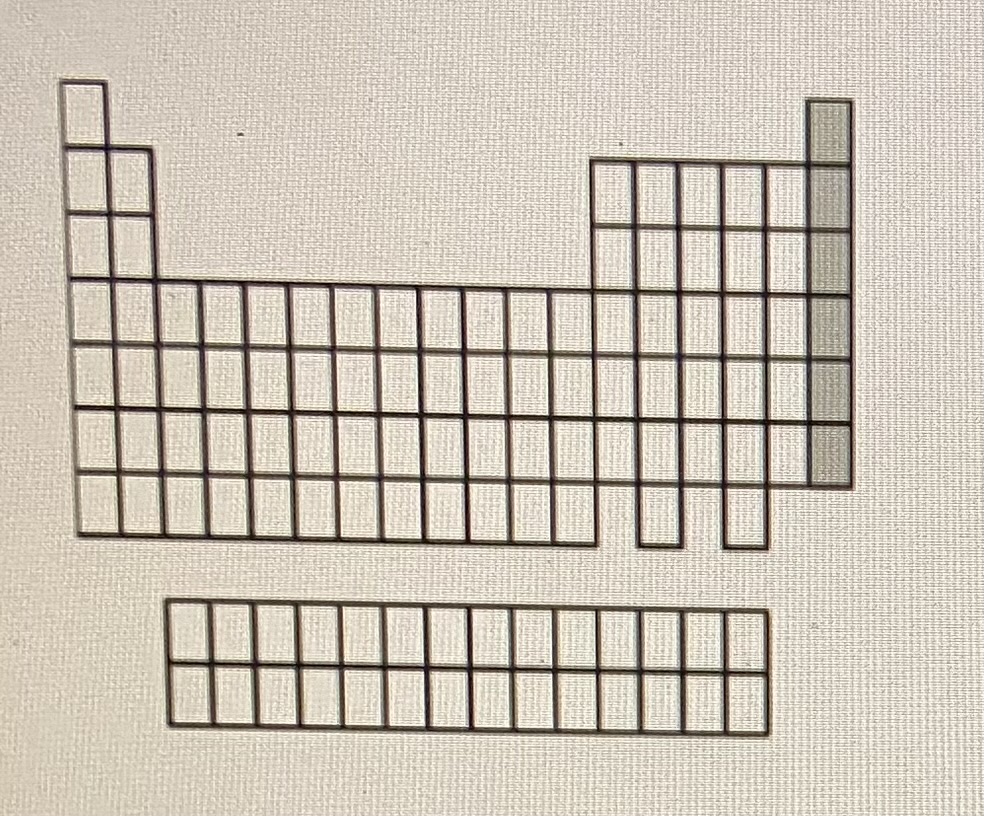
Noble gases
What is the chemical symbol for manganese?
Mn
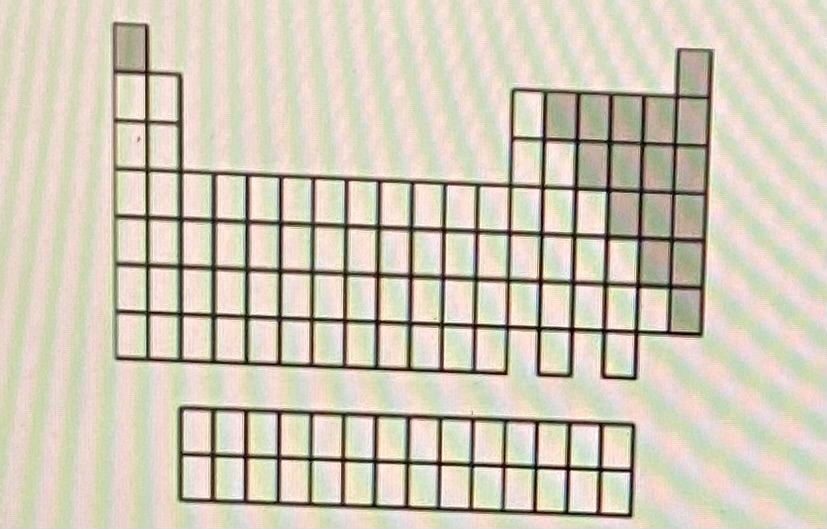
Gases
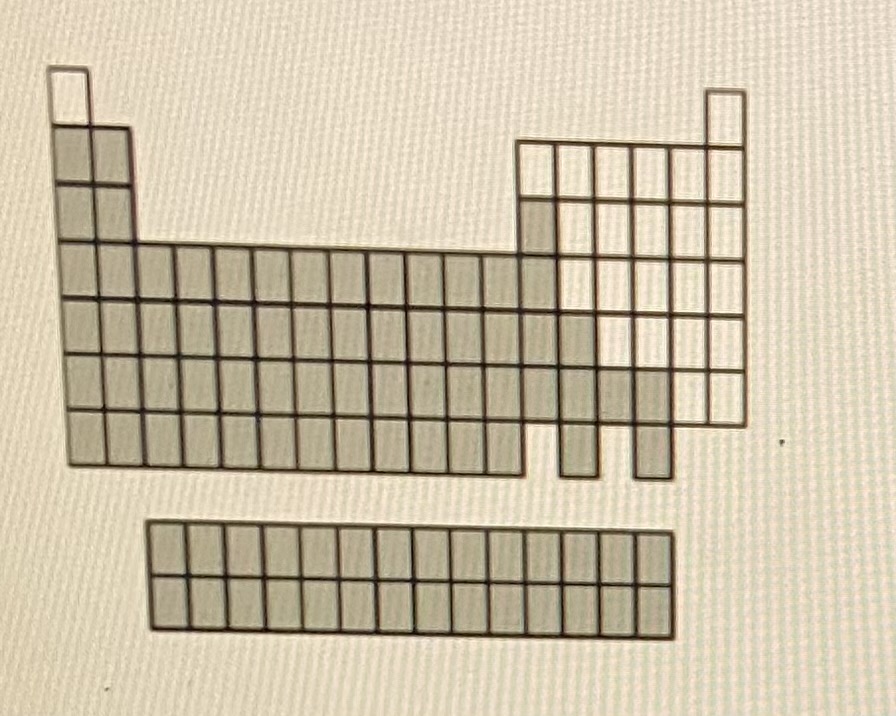
Metals
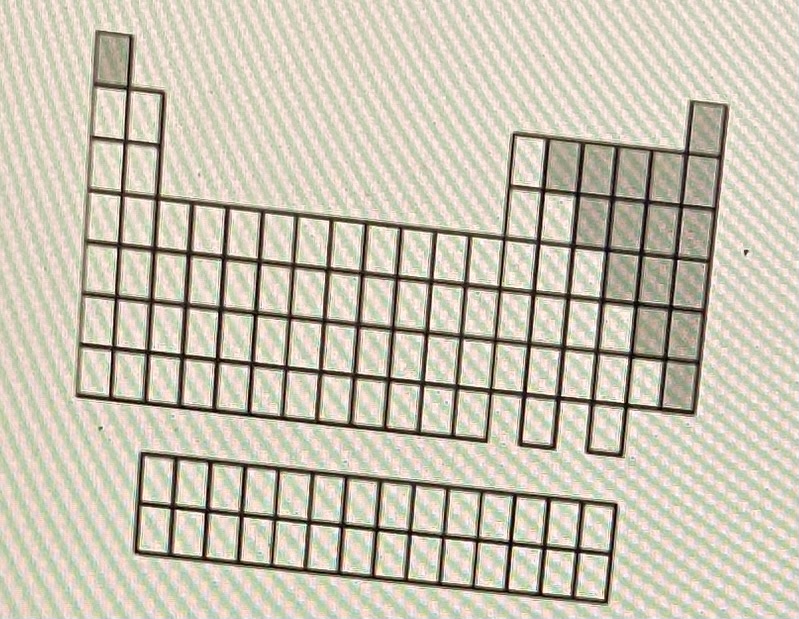
Nonmetals
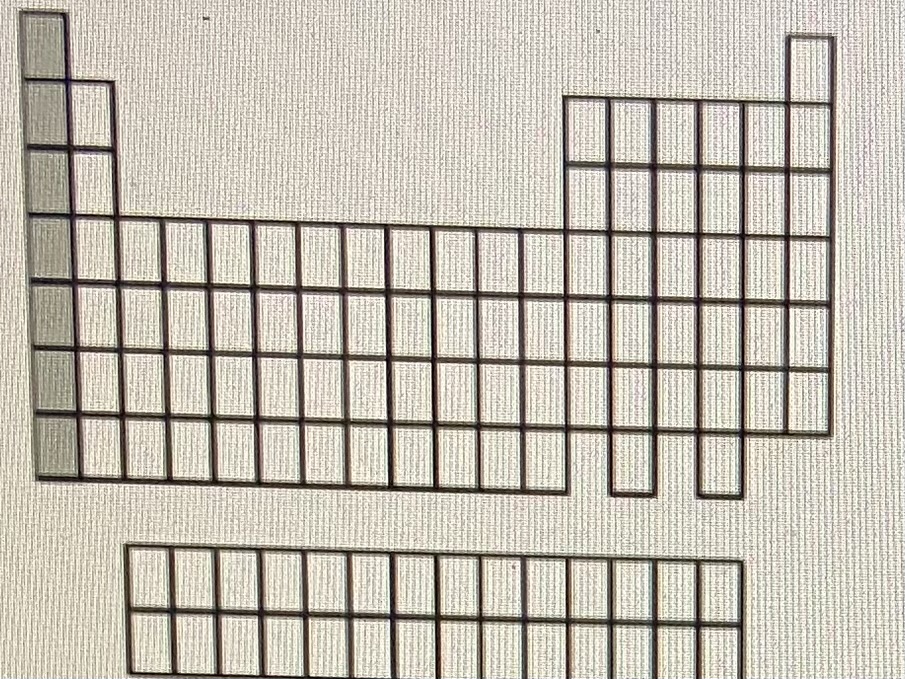
Alkali metals

Semi metals
Poor conductor of heat and electricity
Flourine
Gasses elements chaterogized by low reactivity in group
8A
5A element most metallic
Bi
Platinum chemical symbol
Pt
maganese chemical symbol
Mn
Element for symbols P
Phosphorus