Chapter 1: Matter, Energy, and Measurement
The Study of Chemistry
The Atomic and Molecular Perspective of Chemistry
Chemistry: the study of the properties and behavior of matter.
Matter: the physical material of the universe; anything that has mass and takes up space.
A property is any characteristic that allows us to recognize a particular type of matter and to distinguish it from other types.
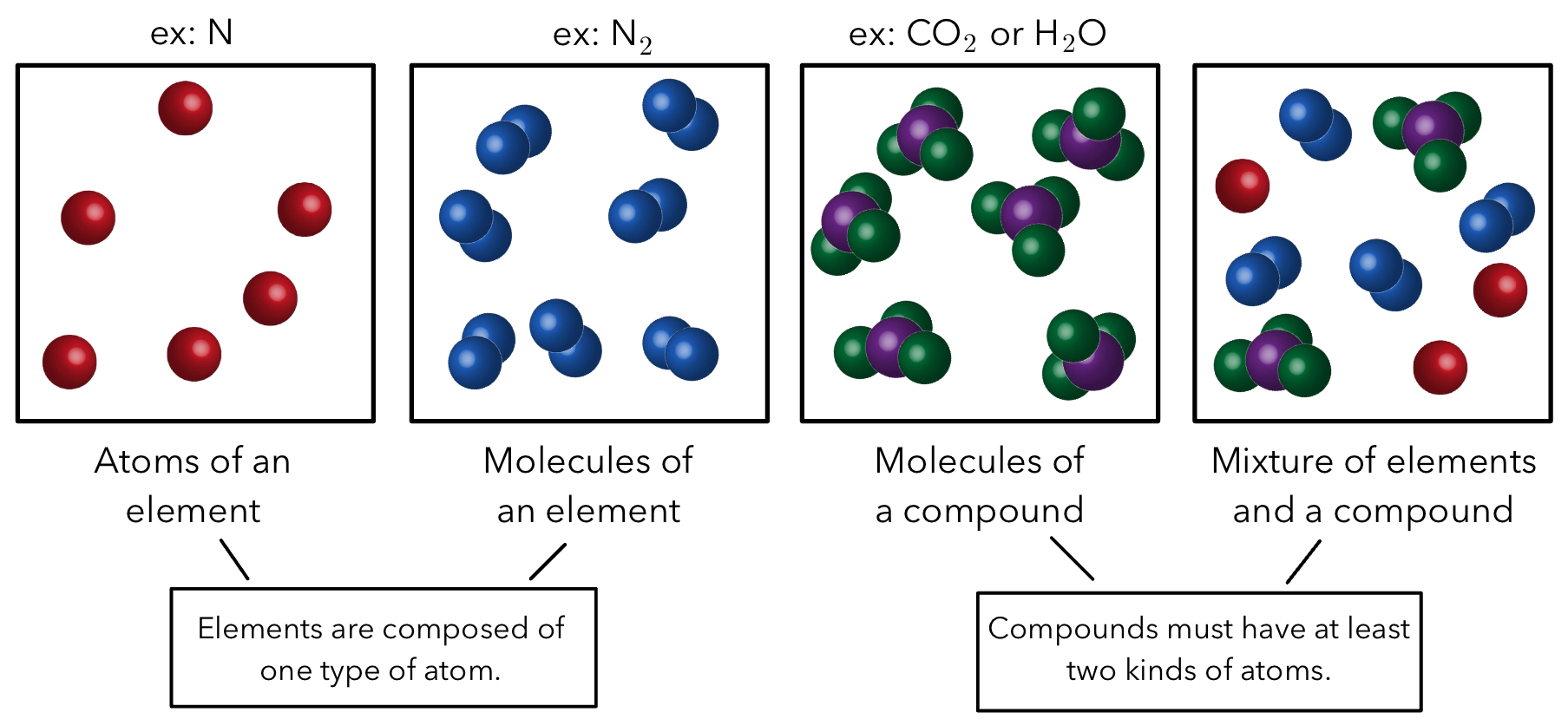
Elements: combine together to create matter.
Atoms: the tiniest particles that are the building blocks of matter and can not be divided further.
Molecules: two or more atoms.
Different molecules can be made from the same elements.
Why Study Chemistry?
Helps improve pharmaceuticals, fertilizers and pesticides, plastics, solar panels, light emitting diodes, and building materials.
Identify harmful chemicals.
Chemists
They do three things:
Make new types of matter, materials, substances, or combinations of substances with desired properties.
Measure the properties of matter.
Develop models that explain and/or predict the properties of matter.
States of Matter
Three states of matter
Solid (s)
Fixed volume and shape.
Molecules packed tightly together.
Example: Ice
Liquid (l)
Fixed volume and shape fits to container.
Closely packed molecules.
Example: Liquid water
Gas (g)
No fixed volume or shape, fits container.
Molecules are far apart.
Molecules can move fast and bounce off container walls.
Open space = less interaction between molecules.
Smaller container = molecules hit each other.
Collisions do not affect shape or volume.
Example: Water vapor
Aqueous (aq)
Solid dissolved in liquid.
States of matter can change through temperature or pressure.
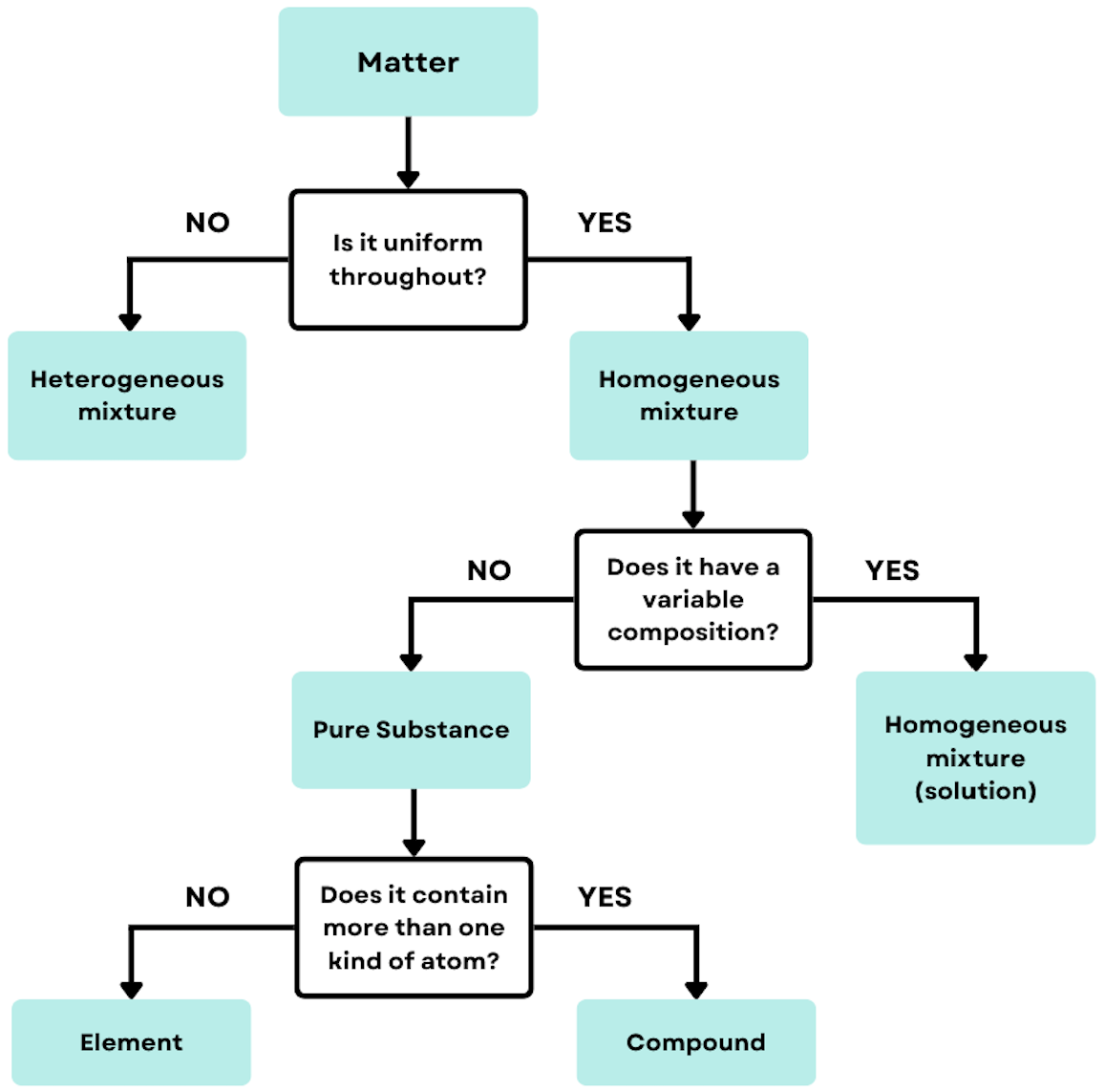
Pure Substances
Pure substance: matter that has distinct properties and a composition that does not vary from sample to sample.
Example: Water and table salt.
The two type of substances are elements and compounds.
Elements
Can’t be decomposed into simpler substances.
Composed of only one kind of atom.
Example: Ar, Be, Xe, C
Compounds
Can be decomposed because it is made up of two or more elements, so the are two or more types of atoms.
Example: CO2, H2O, C2H4
Elements
118 named elements.
Element symbols have one or two letters. First letter capitalized, second lowercase.
Elements are found on the periodic table.
Columns of periodic table have elements with similar properties.
Compounds
Elements can form compounds.
Law of constant composition: states that the elemental composition of a compound is always the same.
Mixtures
Mixtures: combinations of two or more substances in which each substance retains its chemical identity.
Two different types: homogeneous and heterogenous
Matter is made up of mixtures of different substances.
Mixtures can have various compositions.
Components of a mixture are substances making up a mixture.
Heterogeneous mixtures vary in composition.
Do not mix evenly
Examples: Salad, sand in water (does not dissolve), soil
Homogeneous mixtures have uniformed compositions (evenly mixed).
Evenly mix
Aka solutions
Examples: Air, sugar is water (dissolves), steel
Two Types of Properties
Physical properties can be observed without changing the identity and composition of the substance.
Color, odor, density, melting point, boiling point, hardness
Chemical properties describe the way a substance may change, or react, to form other substances.
Flammability
Intensive properties do not depend on the amount of sample being examined and are particularly useful in chemistry.
Temperature, melting point
These are important for identifying a substance.
Extensive properties depend on the amount of sample. Relates to the amount of substance present.
Mass, volume
Physical and Chemical Changes
Physical changes: substance changes physical appearance but not composition.
Example: Cutting a carrot, water changing phases
Changes of state are physical changes.
Chemical change (aka chemical reaction): a substance transforms into a new substance.
Example: Burning, rusting, mixing vinegar and baking soda
Separation of Mixtures
Filtration: separates solids from liquids or gases using a filter.
Distillation: a separation process that depends on the different abilities of substances to form gases.
Chromatography: separates substances on the basis of differences in the ability of substances to adhere to the solid surface, in this case, dyes to paper.
Numbers and Chemistry
Quantitative: numerical measurements
Units of Measurements
SI Units
SI units: preferred metric units for science.
Physical Quantity
Unit Name
Abbrev.
Length
Meter
m
Mass
Kilograms
kg
Temperature
Kelvin
K
Time
Second
s or sec
Amount of substance
Mole
mole (mol)
Electric current
Ampere
A or amp
Luminous intensity
Candela
cd
Metric System
The base units used in the metric system:
Physical Quantity
Unit Name
Abbrev.
Mass
gram
g
Length
meter
m
Time
second
s or sec
Temperature
Degrees Celsius
°C
Temperature
Kelvin
K
Amount of substance
mole
mol
Volume
cubic meter
cm3
Volume
liter
l
Prefixes for Measurements
Different prefixes are used to different values
Prefix
Abbrev.
Scientific Notation
Value
Giga
G
1 x 109
1,000,000,000
Mega
M
1 x 106
1,000,000
kilo
k
1 x 103
1,000
centi
c
1 x 10-2
0.01
milli
m
1 x 10-3
0.001
micro
μ
1 x 10-6
0.000006
nano
n
1 x 10-9
0.000000001
Prefix Examples
Canceled units are shown in pink
Example 1: Convert 0.0077 kg to g
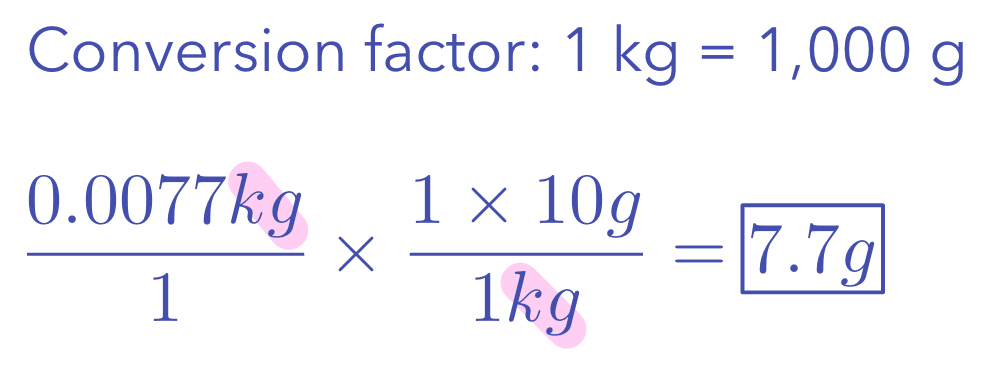
Example 2: Convert 8,800 mL to L
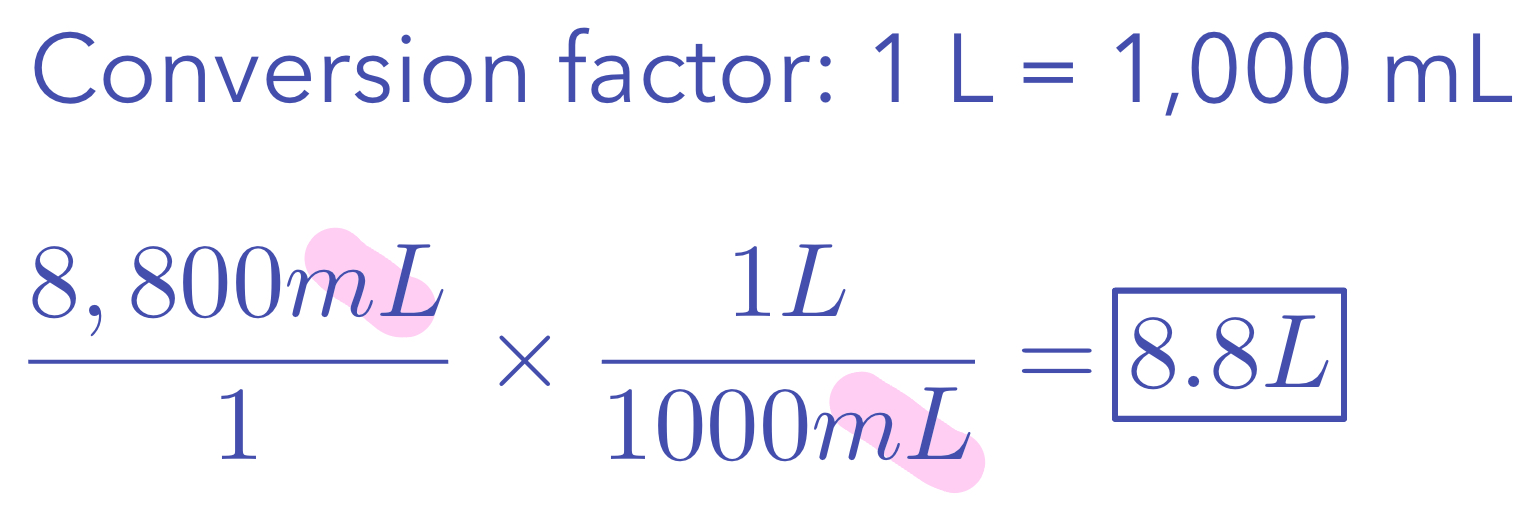
Example 3: Convert 0.00450 cm to nm
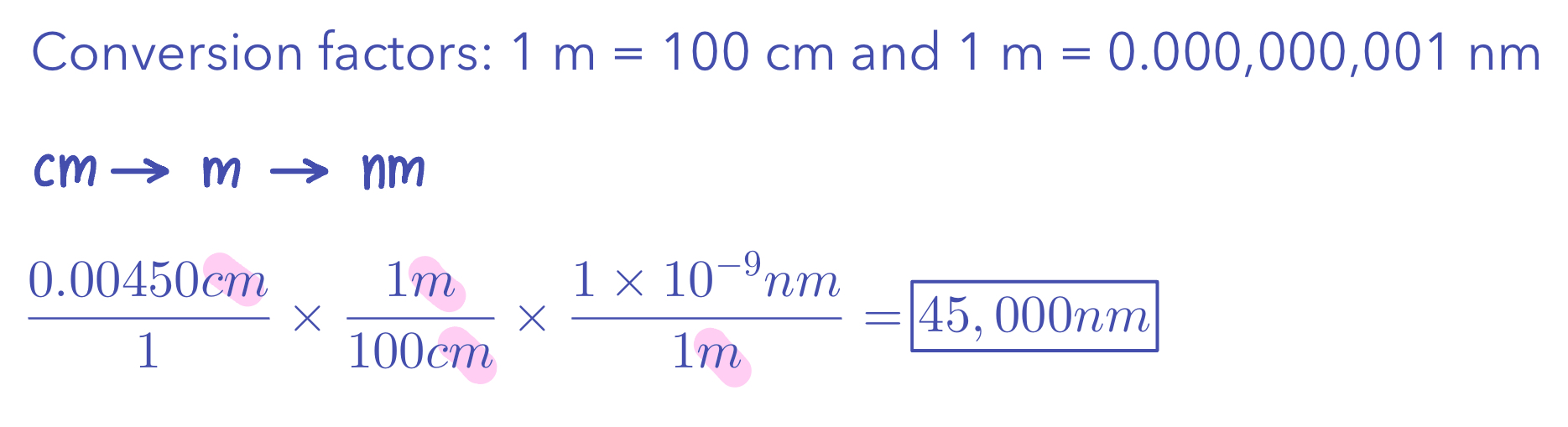
Mass, Length, and Volume
Mass: a measure of the amount of material in an object.
SI unit: kilogram
Length: a measure of distance.
SI unit: meter
Volume: a derived unit from length.
Equation: L x W x H
Common units are the liter and milliliter
Example: cm x cm x cm = cm3
Temperature
Temperature: a measure of the hotness or coldness of an object, is a physical property that determines the direction of heat flow.
SI unit: Kelvin
Absolute zero is equivalent to:
0 K = -273.15 C
Equation for converting Celsius to Kelvin
K = C+273.15
Equation for coverting Celsius to Fahrenheit
F=\dfrac{9}{5}\left( C+32\right)
Equation for converting Fahrenheit to Celsius
C=\dfrac{5}{9}\left( F-32\right)
Density
Density: the amount of mass in a unit volume of a substance.
Most common SI units: g/mL or g/cm3
Equation for density → density = mass/volume
d=\dfrac{m}{v}
Density Examples
Canceled units are shown in pink
Example 1: Find the density of an object using the given information.
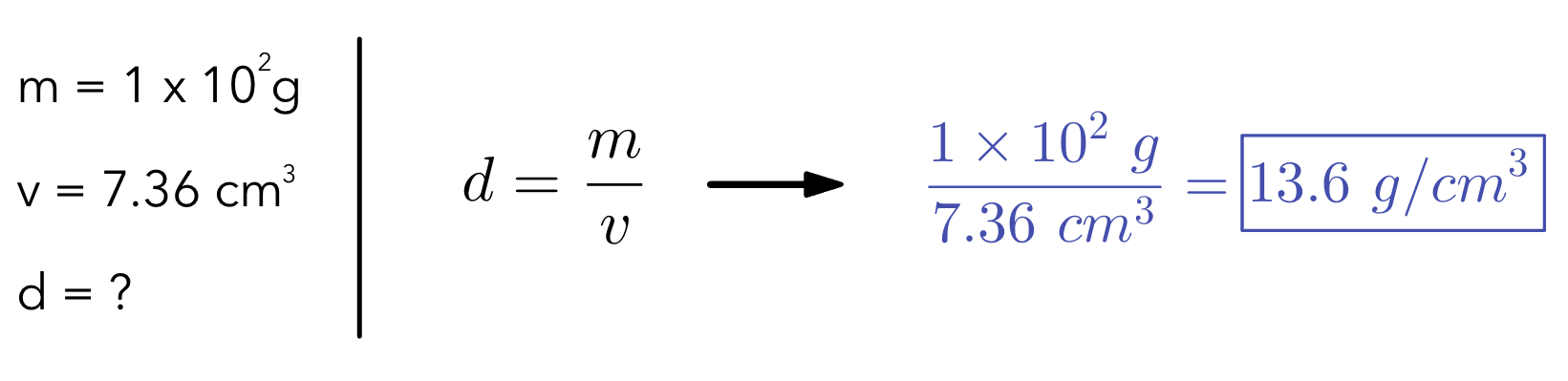
Example 2: Find the volume of an object using the given information.
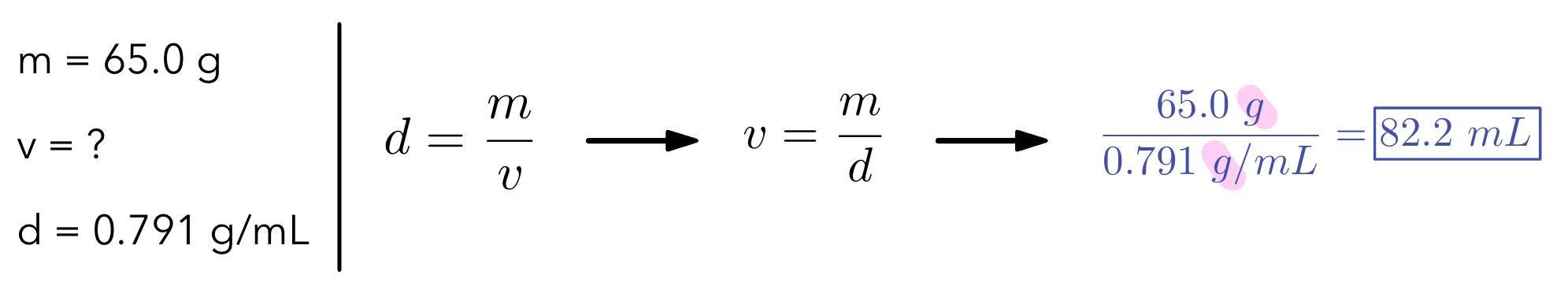
Example 3: Find the mass of an object using the given information
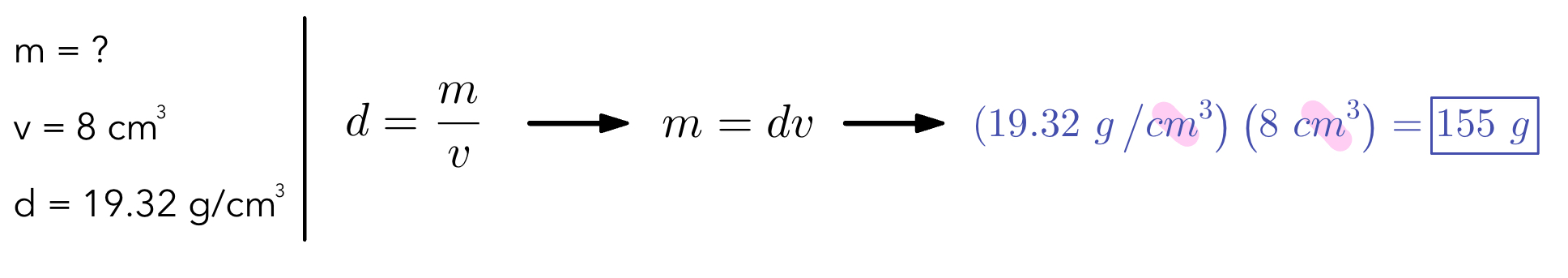
Numbers in Science
Exact numbers: exact values
Defined values
Examples: 12 eggs in a dozen, 12 inches in 1 foot
Inexact numbers: values of some uncertainty
Numbers from measurements.
Uncertainties always exist in measured quantities.
May be inexact from errors (equipment or human errors).
Examples: blood pressure, weight, height
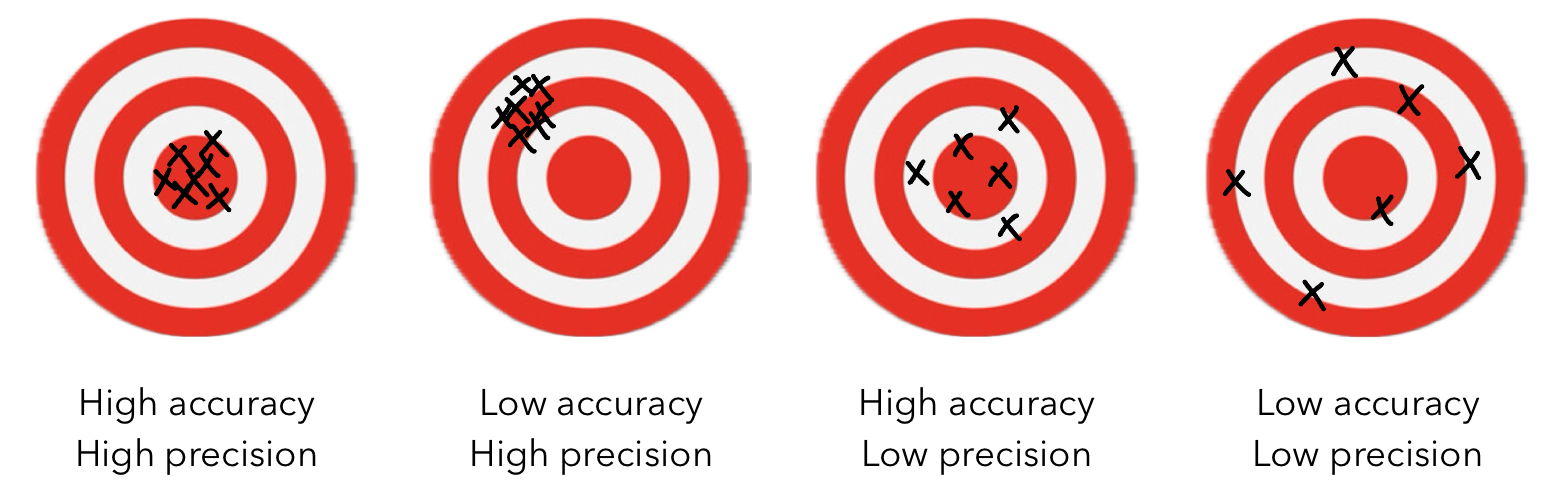
Accuracy vs Precision
Precision: a measure how closely individual measurements agree with one another.
Accuracy: how closely individual measurements agree with the correct or “true” value.
Experimentally, we take several measurements and determine a standard deviation.
Significant Figures
Significant figures: all digits of a measured quantity.
The greater amount of significant figures, the more precise the measurement is.
What numbers are significant:
All non-zeros
Zeros between non-zeros
Zeros at the end if theres a decimal point
Zeros at the beginning of a number are NEVER SIGNIFICANT.
Adding and subtracting significant figures
The answer has the same number of decimal places as the measurement with the fewest decimal places.
20.42 + 1.322 + 83.1 = 104.842
20.42 = two decimal places
1.322 = three decimal places
83.1 = one decimal place!!
Answer: 104.8 (one decimal place)
Multiplying and dividing significant figures
The answer has the same number of significant figures as the measurement with the fewest significant figures.
(6.221)(5.2) = 32.3492
6.221 = four significant figure
5.2 = 2 significant figures!!
Answer: 32 (2 significant figures)
Significant Figures (SF) Examples
Example 1: 73.000 has 5 SF
Example 2: 1400 has 2 SF
Example 3: 0.005090 has 4 SF (the zeros before 5 do not count)
Example 4: 6.378 × 108 has 4 SF
Example 5: 390200 has 4 SF (the zeros after 2 do not count, there is no decimal point)
Dimensional Analysis
In dimensional analysis, units are multiplied together or divided into each other along with the numerical values.
Equivalent units cancel out.
Conversion Factors
Conversion factor: a fraction whose numerator and denominator are the same quantity expressed in different units.
Examples:
1 foot/12 inches = 12 inches/1 foot
Denominator is used to cancel units.
Given unit x Desired unit/Given unit
Given unit cancels
Two or more conversion factors:
First conversion cancel given unit.
Following conversions cancels another unit and gives desired.
Know that 1 mL = 1 cm3
Units of Energy
The SI unit for energy is the joule (J).
A larger SI unit used is the kilojoule (kJ).
A calorie (cal) is a non-SI unit that is the amount of energy required to raise the temperature of 1 g of water by one degree Celsius.
1 cal = 4.184 J
1 Cal = 1000 cal = 1 kcal
Reference: Chemistry The Central Science (14th Edition)