IB CHEM- STRUCTURE 1
1/111
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
112 Terms
Pure substances
Made of only one type of substance
Have a fixed composition
Mixtures
Combination of 2+ pure substances
NO fixed composition
2+ different elements or compounds NOT chemically combined
Can be separated by physical methods (separation techniques)
Keep the properties of the individual elements of compounds
Alloys are included even though they contain metallic bonds
Homogenous [mixture]
no visible phases or boundaries
Ex. Saltwater
uniform: different parts are equally distributed and in the same state
Heterogeneous
visible phases or boundaries
Ex. Oil and water
different parts of the mixture have different compositions or states
Elements
simplest form of matter
One type of atom
Cannot be chemically broken down into simpler substances
Ex. Iron (Fe)
Compounds
2+ different elements chemically combined in a fixed ratio
Cannot be separated by physical methods
Ex. Methane, CH4
properties of compounds are much different from those of individual elements
Solute
A substance (usually solid) dissolved in a solution
Solvent
A substance (usually liquid) in which other substances are dissolved
Solution
A homogenous mixture composed of a solute dissolved in water (the solvent)
Dissolve
When solute particles are surrounded by solvent particles
Solubility
The ability of a substance to dissolve into a solvent to form a solution
Insoluble
When a solute does not dissolve in a solvent
Soluble
Can dissolve in a solvent to produce a solution
Filtrate
A substance that has passed through a filter
Residue
The insoluble component (usually a solid) of a mixture that remains on the filter paper after filtration
Volatility
The tendency of a substance to undergo evaporation
Distillate
The part of a liquid mixture that evaporates and condenses in the distillation process
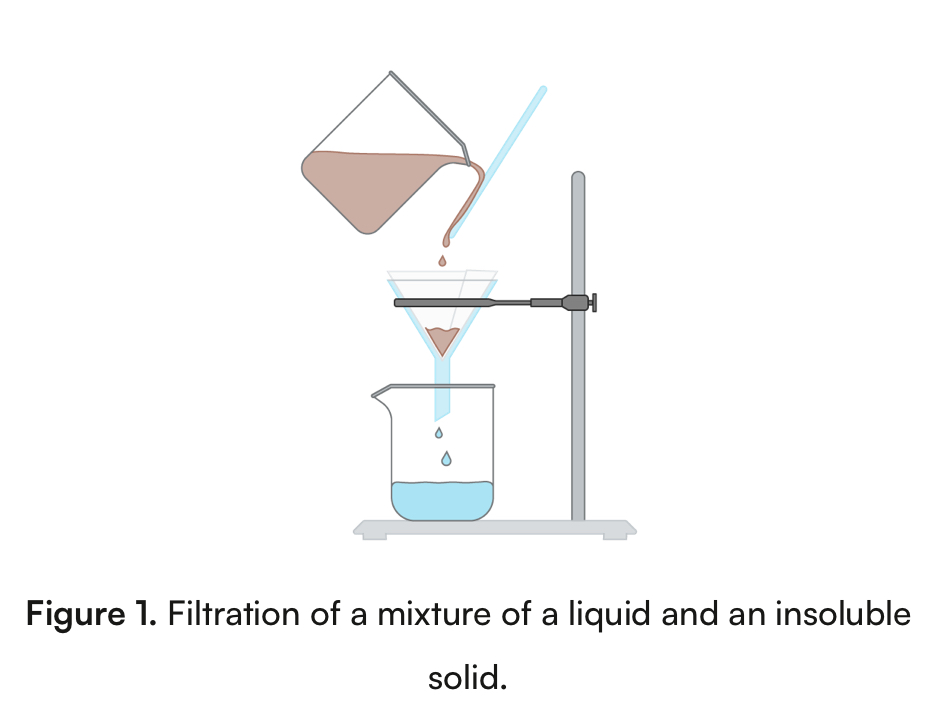
Filtration
Physical property: particle size
separates an insoluble solid from a liquid
Heterogenous mixture
Ex. Salt and vinegar
Large particles get stuck on filter paper, small pass through
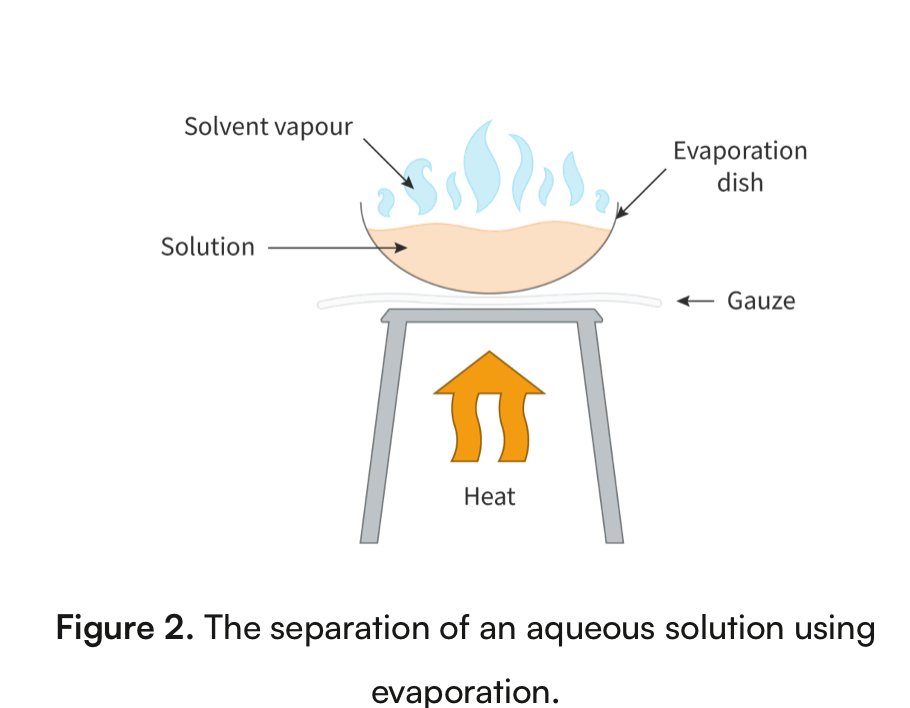
Evaporation
Physical property: boiling point
separates a dissolved solid from a liquid
Homogeneous mixture
Ex. Salt and water
Liquid evaporates leaving the solid behind
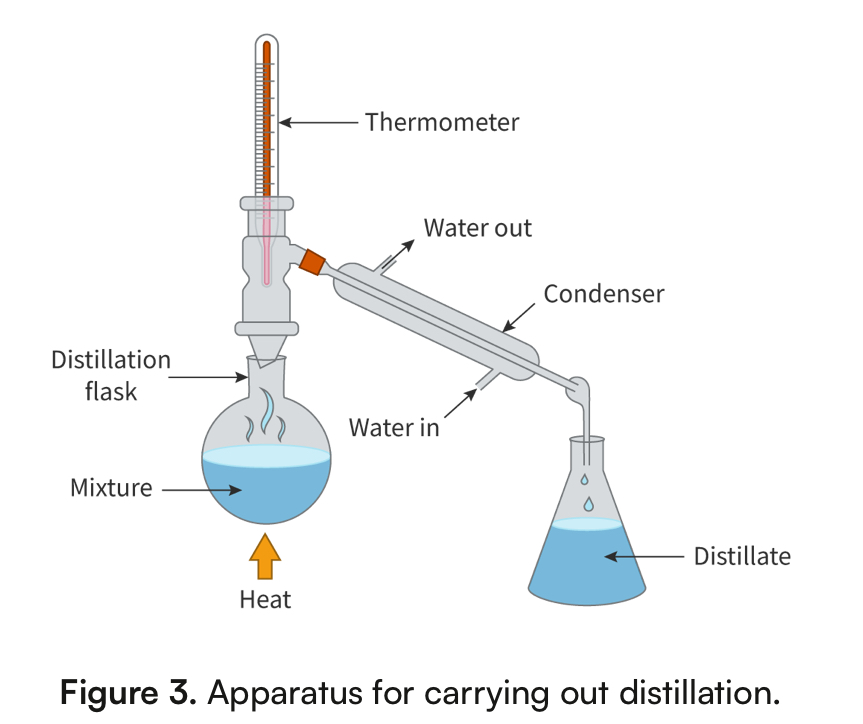
Distillation
Physical Property: boiling point
separates two liquids
Homogeneous mixtures
Ex. Water and ethanol
One liquid evaporates first, then condenses; pours into a separate container
Recrystallization
Physical property: solubility at different temperatures
separates impurities from a solid
Ex. Purify sugar crystals
Impure mixture is dissolved in hot liquid; as it cools, pure crystals form and impurities stay dissolved; use filtration to separate crystals from impurities
Least-soluble solution will crystallize first
Chromatography
another type of separation technique used to separate mixtures that contain very small amounts of each component, or to determine how pure a substance is
set ups contain 2 phases
Mobile phase- moves
Stationary phase- stays in place
Works due to components of a mixture having different tendencies to either absorb onion a stationary surface or dissolve into a mobile solvent
2 types: paper chromatography and thin-layer chromatography (TLC)
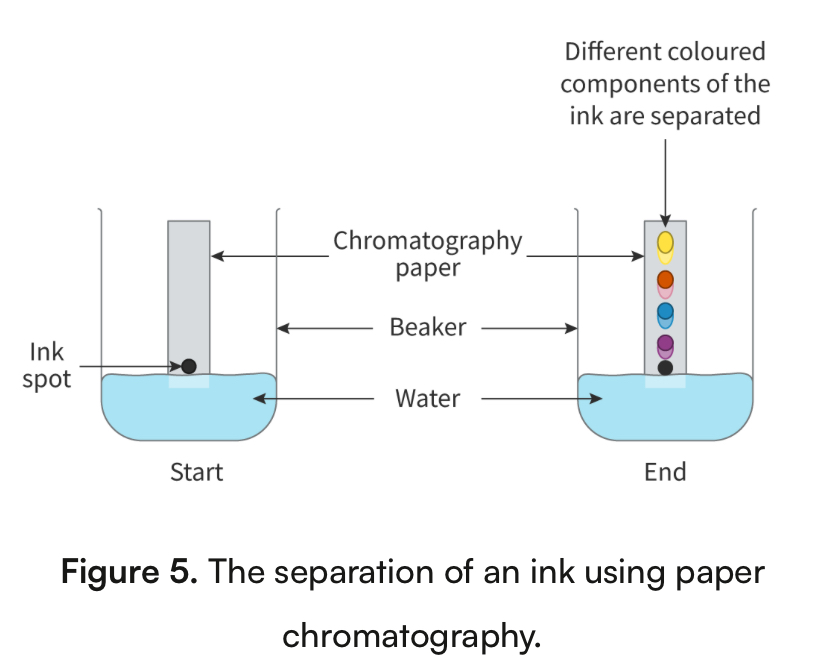
paper chromatography
a mixture is applied to a piece of chromatography paper
Paper is in stationary phase
Solvent is in mobile phase
As the mobile phase starts to climb up the paper, the mixture will be carried with it each at a different rate
Components that have a great affinity for the mobile phase will move father up the paper because they will interact with the mobile phase more
Components that have a great affinity for the stationary phase will move less far up the paper because they will interact with the stationary phase more
Thin-Layer Chromatography (TLC)
very similar to paper chromatography
Advantage: separated components can be recovered pure
Kinetic Molecular Theory: States of Matter
Matter is composed of particles (if an element) or molecules (if a compound). These particles have kinetic energy (motion energy) so they are constantly moving
Higher temperature= more movement; more likely to be a gas (straight line motion)
Lower temperature=less movement; more likely to be a solid (vibrational motion)
Collision between particles are elastic (no loss in kinetic energy)
Solid-s
fixed volume
Fixed shape
Cannot be compressed
Strong attractive forces between particles
Particles vibrate in fixed positions it do not move around
More dense
Have least amount of kinetic energy
Liquid- l
fixed volume
No fixed shape
Cannot be compressed
Weaker attractive forces between particles
Particles vibrate, rotate, and move around
Less dense
WILL TAKE THE SHAPE OF THE BOTTOM OF THE CONTAINER BUT NOT OF THE CONTAINER ITSELF
Gas-g
no fixed volume
No fixed shape
Can be compressed
Negligible attractive forces between particles
Particles vibrate, rotate and move fast
Least dense
Have the most amount of kinetic energy
Aqueous- aq
a solid is dissolved in H2O
Each molecule or ion is surrounded by water
Solvation
The separation of a heterogeneous mixture of two solids based on difference in solubility if one of the substances is soluble in a solvent, but the other solid is insoluble.
during solvation, the solvent molecules (most often water) surround the soluble molecules and dissolve the solid into a solution
Th insoluble solution can now be separated by filtration
The soluble substance can be separated from the solution by evaporation
Plasma
An ionized gas mainly found in outer space
Density
Mass per unit volume
substances with higher densities will feel ‘heavier’ compared to substances with lower densities (of the same volume)
Formula: d=m/v
Solids ten times have higher densities than liquids which tend to have higher densities than gases
Changes of state or phase changes
Occur when a substance changes from one physical state to another
a physical change because it is not chemically changing
Reversible processes
Sublimation
Solid → gas
dry ice (solid CO2) sublimes from a solid directly to a gas
Absorbed released
Deposition
Gas→ solid
formation of frost in a freezer as the moisture in the air forms solid ice
Released heat
Evaporation/ vaporization
Liquid→ gas
takes place only at the surface of the liquid
Can occur at temperatures below boiling point of the liquid
Absorbed heat
Melting
Solid→ liquid
Absorbed heat
Freezing
Liquid→ solid
Released heat
Condensation
Gas→ liquid
Released heat
Celsius scale
Based on the freezing point of water (0degrees Celsius)and the boiling point of water (100degrees Celsius)
Kelvin scale
An absolute temperature scale where the lowest possible value is 0K, known as absolute zero
at absolute zero, particles have zero kinetic energy
The temperature in kelvin is directly proportional to the average kinetic energy of the particles in the substance
does not have negative temperatures
Converting between K and degrees C
Add or subtract 273 depending on which scale you are converting from
C to K- add 273
K to C- subtract 273
Heating and cooling curves
Changes of state graphed by producing a heating (or cooling) curve
Shows how the state of matter changes as heat is added; cooling curve would be the opposite, starting at a gas and ending with a solid with the temperature decreasing
there are some points where the temperature remains constant because all the added heat is being used to overcome the intermolecular forces that act between the particles
Nucleons
Located in the nucleus
Protons
Relative mass of 1 amu
Charge of +1
Neutrons
Relative mass of 1 amu
No charge
Electrons
Located in the electron cloud outside nucleus
Relative mass of 1/2000
Negative charge
Atomic number
The number of protons in the nucleus of an atom
Mass number/ nucleon number
The number of protons and the number of neutrons in the nucleus of an atom
Nuclear symbol notation
Used to represent an element and can determine number of protons, neutrons, an electrons in an atom or ion
Top number is mass number
Element symbol- center large
Bottom number is atomic number
Ions
A charged particle
Has a charge as the number of protons do not equal the number of electrons
Positive ions
Formed when atoms lose electrons
Has fewer electrons than protons
Negative ions
Formed when atoms gain electrons
Has more electrons than protons
Isotopes
Atoms of the same elements that have different number of neutrons
Relative atomic mass
The weighted average mass of an atom compared to 1/12 of the mass of the carbon-12 atom
Calculated from the percent abundance and the masses of the isotopes of an atom:
mass # of each isotope is multiplied by its percent abundance, those values are added together and then divided by 100
Spectroscopy
The study of interaction between matter and light
emission spectra
The range of frequencies or wavelengths of electromagnetic radiation emitted during an electron transition from a higher to a lower energy level
Electron transitions
The movement of an electron between the energy levels in an atom, accompanied by the absorption or emission of energy
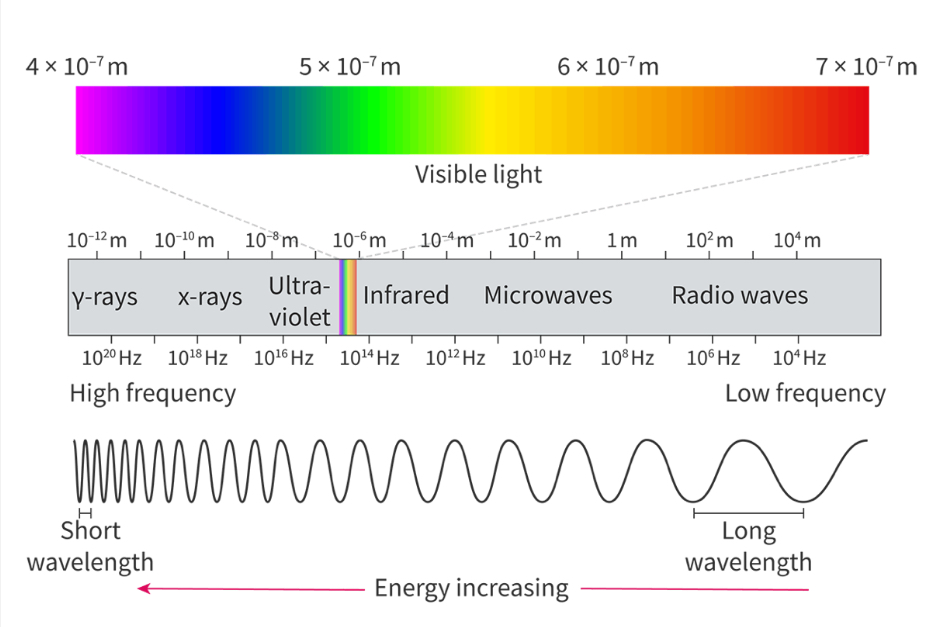
The electromagnetic (EM) spectrum
Divided into 7 regions arranged in order of frequency, wavelength or energy
Frequency and wavelength are inversely proportional
The energy and frequency are directly proportional
Radio waves have the lowest energy, lowest frequency, and longest wavelength
Gamma rays have the highest energy, highest frequency and shortest wavelength
Wavelength λ
The distance between two crest in an an oscillating wave
Units of distance (m)
Frequency( f)
The number of waves that pass a point in one second
Units: hertz (Hz) or s-1
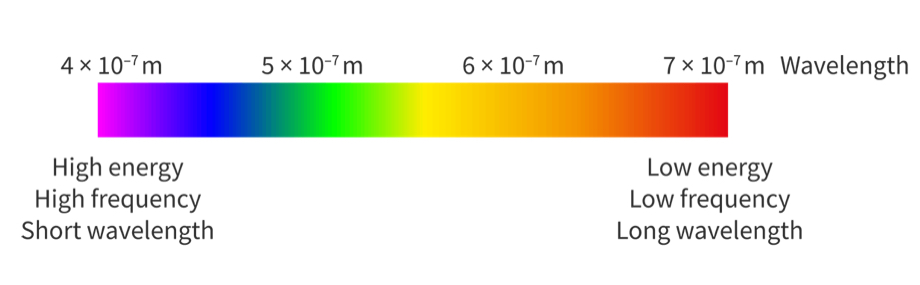
Continuous spectrum
A spectrum that contains all the frequencies (or wavelengths) across a range of electromagnetic radiation
our eyes see the continuous spectrum as white light
Emission line spectrum
The range of frequencies or wavelengths of electromagnetic radiation emitted during an electron transition from a higher to a lower energy level
lines get closer together (converge) at high energy, which corresponds to high frequency and short wavelength → the distance between the blue and violet lines on the hydrogen emission spectrum is smaller than the distance between the red line and light blue line
Continuous spectrum vs emission line spectrum
a continuous spectrum shows all the wavelengths or frequencies of visible light from red to violet
An emission line spectrum only shows specific wavelengths or frequencies of light. These are shown as colored lines on a black background
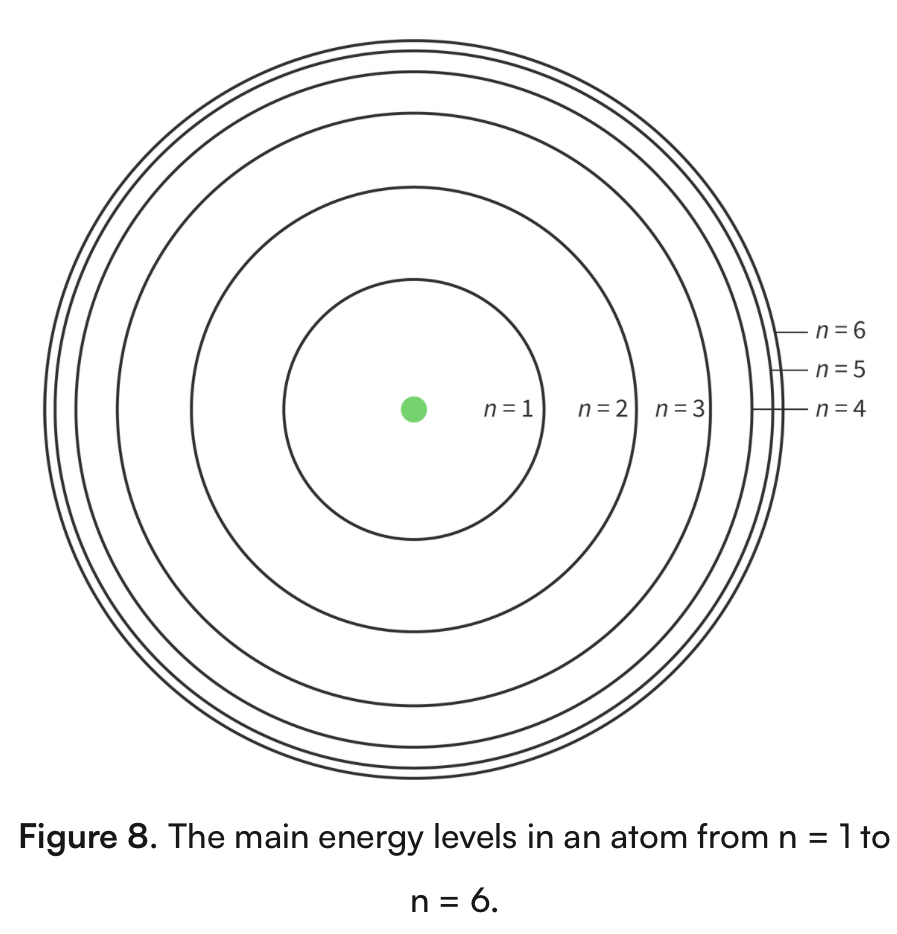
Bohr model of the atom
An atomic model that shows energy levels at fixed distances from the nucleus
Principal quantum number
The main energy level occupied by electrons, assigned the letter n
n=1 is closest to the nucleus (known as ground state)
As the value of n increases, the distance from the nucleus and its energy increases
N=1 has the lowest energy n= has the highest energy
Main energy levels converge at high energy
Photons
An elementary particle of discrete amounts of electromagnetic radiation
Electrons transitioning between the energy levels
By either absorbing or emitting energy
the energy absorbed or emitted is in the form of photons (small packets of energy)
If an electron absorbs a discrete or an exact amount of energy, it will transition from a lower energy level to a higher energy level, for example from n = 2 to n = 3.
The electron is now said to be in an excited state after absorbing (The excited state is unstable relative to the ground state.)
The unstable electron emits the same amount of energy that it absorbed, and it transitions back down to n = 2.
The amount of energy emitted by the electron in the transition from n = 3 to n = 2 corresponds to the wavelengths of visible light
A line will be observed on the emission line spectrum
** the amount of energy emitted depends on the size of the transition (more energy is emitted for farther distance)
Sublevels
The smaller division of main energy levels, assigned to letters s,p,d,
based on the shape of atomic orbitals
Recap: main energy levels are divided into sublevels which are made up of atomic orbitals
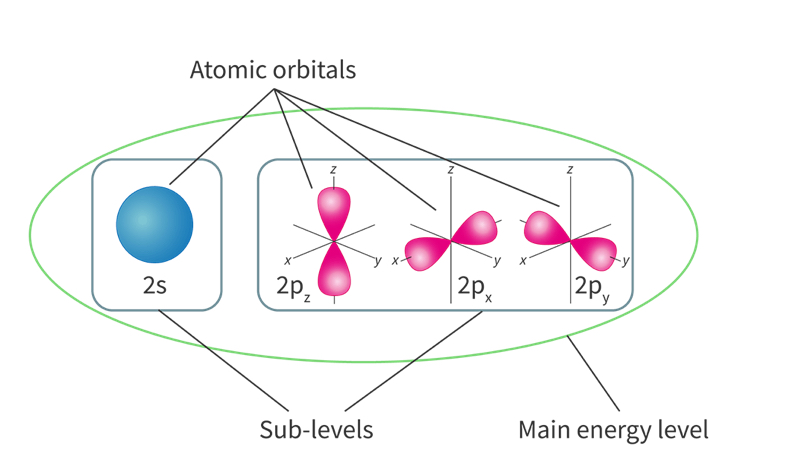
Atomic orbitals
A region of space where there is a high probability of finding an electron
a single atomic orbital can hold a maximum of 2 electrons with specific orientations
S sublevel
spherical
Only consists of a single s atomic orbital so it I can hold maximum of 2 electrons
P sublevel
composed of three p atomic orbitals
Can hold a maximum of 6 electrons
D sublevel
complex shape
Contains 5 d atomic orbitals
F sublevel
Complex shape
contains 7 atomic orbitals
Maximum number of electrons in an energy level
2n2 where n is the principal energy level number
Isoelectronic
Different ions of different elements with the same electron configuration
ex. Mg2+ and Na+
Electron configuration
Shows the arrangement of electrons in their different levels around the nucleus of an atom
For ions, take away or add the number of electrons at the end
Aufbau principle
States that when adding electrons to an atom, the lower energy orbitals must be filled first
Pauli exclusion principle
States that an atomic orbital can only hold two electrons and they must have opposite spins
Hund’s rule
States that when we have degenerate orbitals (orbitals of the same energy) then each orbital is filled with single electron before being double occupied
exceptions to orbital diagrams and electron configurations
Cr and Cu
orbitals or sublevels want to be completely full OR half-full
in Cr: one electron in 4s goes to 3d so BOTH are HALF-FULL→ more stable
Assumptions made in the ideal gas model
the particles in a gas are in constant, random, straight-line motion
collisions between the particles are elastic(no energy lost)
intermolecular forces between the particles are insignificant/negligible
The average distance between gas molecules is much LARGER than the size of the molecules→ gas particles have negligible volume
the average kinetic energy of the particles in a gas is directly proportional to the absolute temperature (in kelvin)
2 conditions under which real gases deviate the most from ideal gas behavior
A high pressure
gas particles are closer together and are influenced by the forces of attraction
At low temperatures
particles move less rapidly and therefore have less kinetic energy, so there is a greater opportunity for intermolecular forces between the particles to have an effect
ideal gas behavior
low pressure
gas particles are far apart
forces of attraction are negligible
high temperature
less opportunity for intermolecular forces between particles to have effect
particles move more rapidly (more kinetic energy)
preparing a standard solution procedure steps
measure mass of solute using a mass balance
Dissolve solute in a small volume of distilled water in a beaker and mixing with a stirring rod
Transfer to volumetric flask via funnel
Rinse beaker, stirring rod and funnel with distilled water
Add distilled water to the volumetric flask until the bottom of the meniscus is at the 500 cm³ mark
Add stopper. and invert to mix thoroughly
Molar mass of ideal gas formula
M=mRT/PV
SI units for ideal gas equations
Pressure- Pa
Temperature- K
Volume- m3
mole
the number of particles present in exactly 12 g of the carbon-12 isotope. this is equal to the Avagadro constant: 6.02 × 1023 particles
formula unit
the empirical formula for an ionic compound that represents the simplest ratio of ions making up the compound
SI
the International System of Units
relative formula mass
the mass of a compound relative to 1/12 the mass of an atom of carbon-12
does not have any units
relative molecular mass
the mass of one molecule of a molecular compound, measured by comparison to 1/12 the mass of an atom of carbon-12
does not have units
molar mass
the mass of one mole of a substance,
units: g mol-1
mole equation
n=m/M
n-number of moles
m-mass
M- molar mass
Law of Definite Proportions
For any given compound, the ratio of constituent elements is fixed
also known as law of definite composition or Proust’s law
percent composition
the relative amount of each element in a compound
will only give the simplest ratio of atoms in a compound (empirical formula)
empirical formula
the simplest ratio of atoms in a compound
molecular formula
the actual number of atoms in the compound
concentration
the number of particles or moles in a given volume, mol dm-3
concentrated and dilute are qualitative terms to describe concentration
concentration: mass per volume
C=m/v
C- concentration: g dm-3
m-mass of solute:g
v-total volume of solution dm3
concentration: in terms of moles per unit volume
C=n/v
C- concentration: mol dm-3
n-moles of solute: n
v-total volume of solution: dm3
Factors that affect the number of particles of gas in a sealed container
the volume of the container
larger the container, the more particles can fit in
the temperature of the gas
greater temperatures mean more kinetic energy and therefore greater speeds the particles will travel
at low temperatures, the particles will move slowly, so more particles fit inside the container
at high temperatures, the particles need more space to move, therefore fewer particles can be accommodated
the pressures of the gas
at low pressures, there are fewer collisions with the sides of the container because there are fewer particles present
when more particles are present, the pressure will be higher
Volume, temperature, and gas determine the number of gaseous elementary particles that can fit in a container