Bohr Models and Electron Configuration
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
31 Terms
Compare and contrast the Bohr Planetary Model and the Schrödinger Quantum Mechanical Model
Bohr has energy levels in set rings/orbitals, Schrödinger has electrons in clouds of probability (still had energy levels, but also sublevels & orbitals)
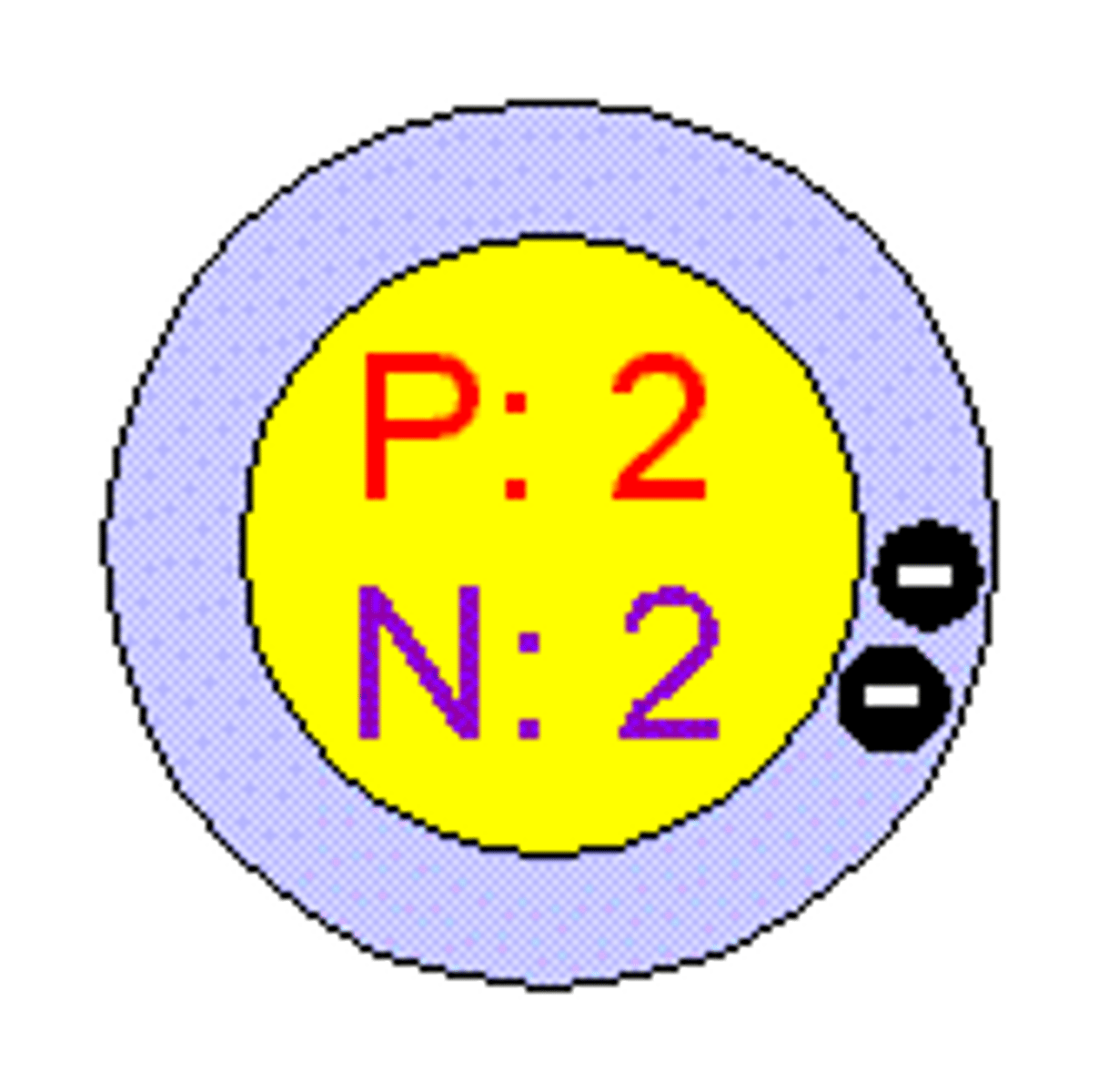
Bohr Model for Helium-4
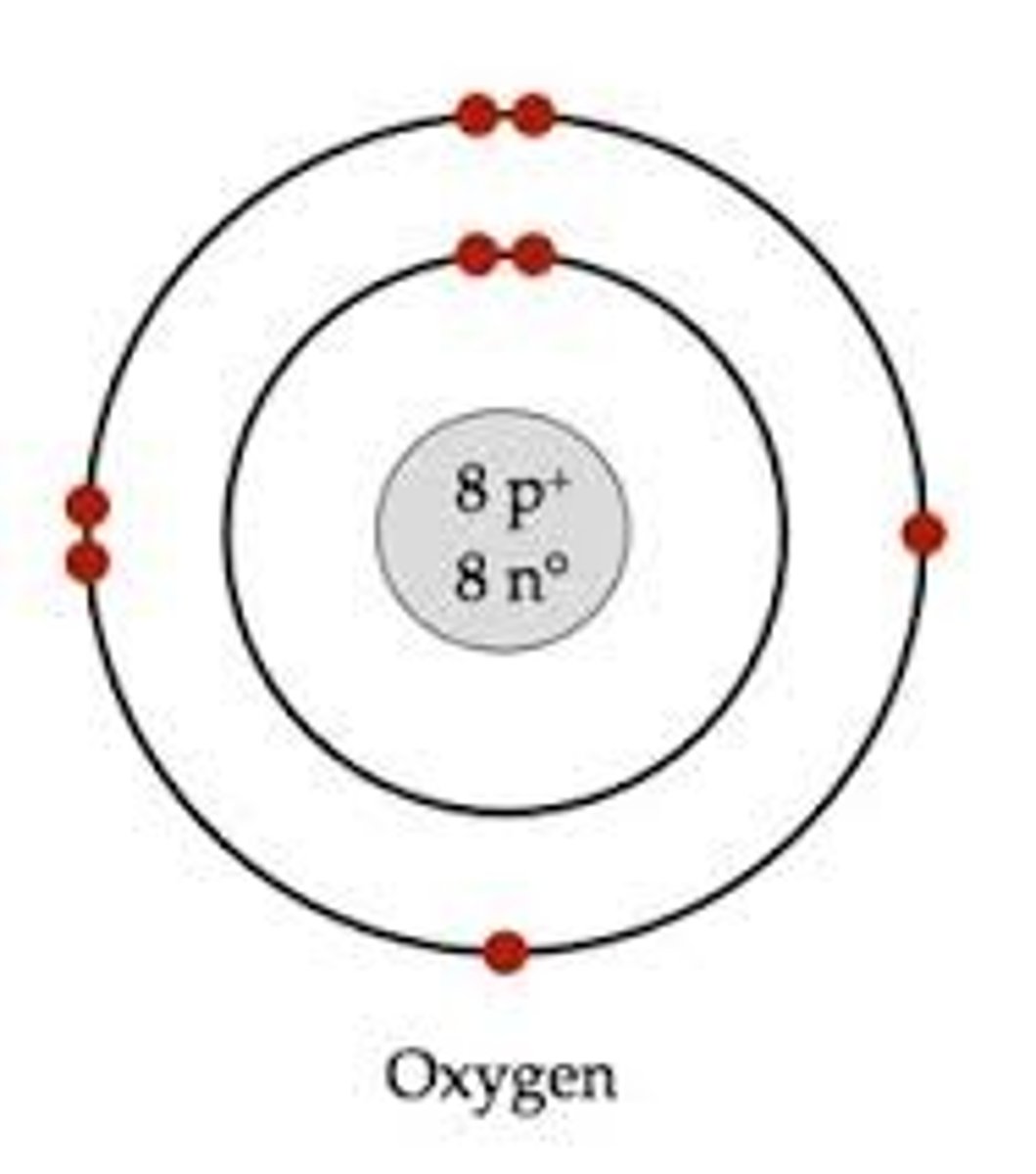
Bohr Model for Oxygen-16
Bohr Model for Silicon-28
14 p+
14 nº
1st level: 2
2nd level: 8
3rd level: 4
Bohr Model for Calcium-40
20 p+
20 nº
1st level: 2
2nd level: 8
3rd level: 8
4th level: 2
How does knowing the "blocks" on the Periodic Table help you determine the configurations?
- Blocks tell you ending configuration for neutral ground state atom
- rows help you determine ending of each energy level
- columns tell you the # of electrons in the ending configuration
What is the ending configuration for every Noble Gas (except He)?
Ne: 2p6
Ar: 3p6
Kr: 4p6
Xe: 5p6
Rn: 6p6
How many electrons can each sublevel hold?
s- 2
p- 6
d- 10
f- 14
Aufbau Principle
An electron occupies the lowest-energy orbital that can receive it
Pauli Exclusion Principle
when you have a pair, you have opposite spins
Hund's Rule
spread out e- in a sublevel before you split up
How to find the electron configuration of ions
cation: move 2 columns back
anion: move 2 columns forward
Copper's electron configuration
[Ar] 4s1 3d10
Chromium's electron configuration
[Ar] 4s1 3d5
Why do copper and chromium have a different observed electron configuration than expected?
fully & 1/2 filled sublevels are stable, anything else is not. Chromium alters so both 3d & 4s are filled, copper alters so 3d is full & 4s is 1/2 full.
Where is more practice?
The review in Week 9, warmups
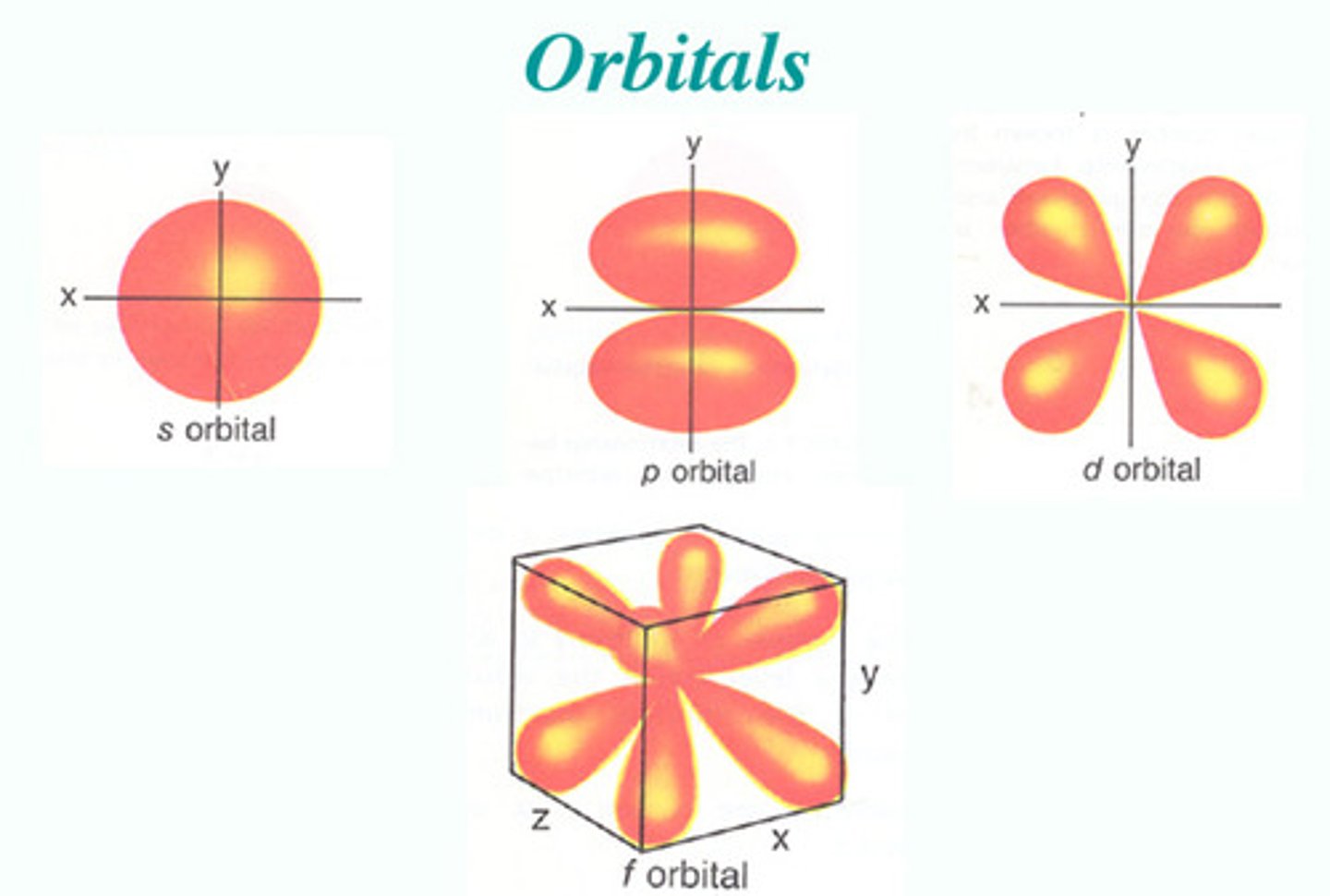
Orbital
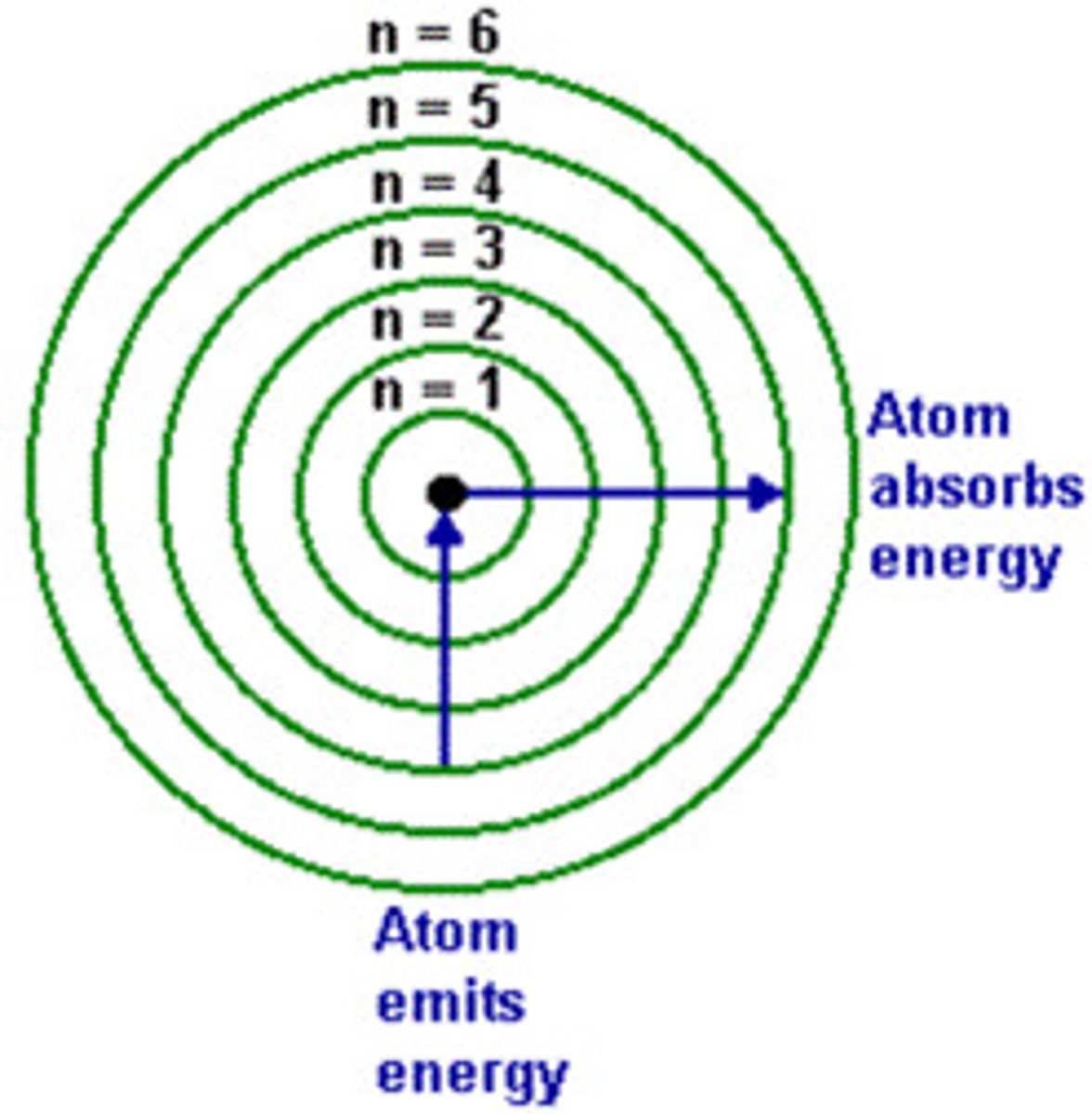
energy level
a region of an atom in which electrons of the same energy are likely to be found
sublevel
An atomic orbital, or collection of atomic orbitals, that occupy a principal energy level and are called s, p, d, and f.
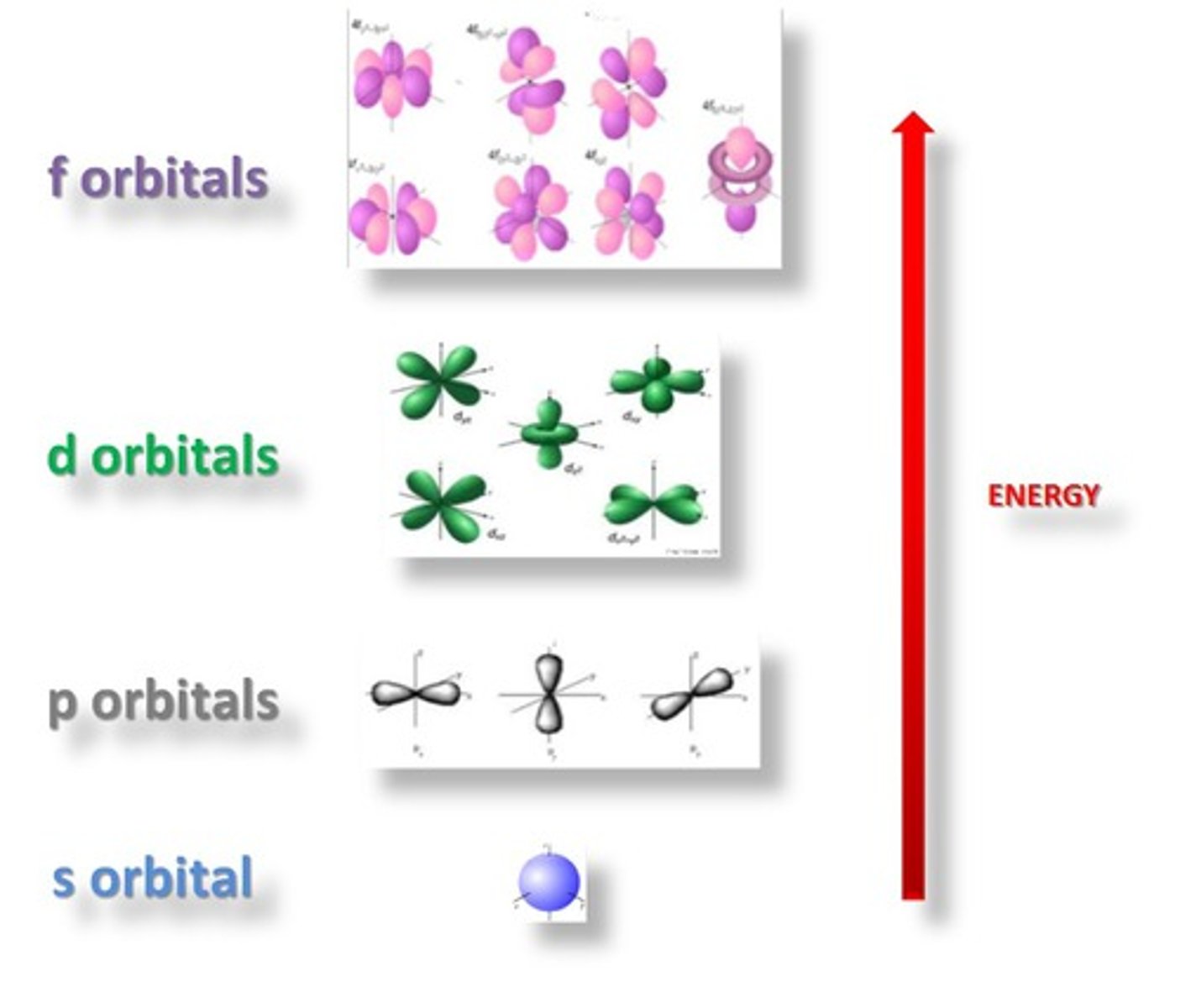
total electrons in the 1st energy level
2
total electrons in the 2nd energy level
8
total electrons in the 3rd energy level
18
total electrons in the 4th energy level
32
total orbitals in the s sub-level
1
total orbitals in the p sub-level
3
total orbitals in the d sub-level
5
total orbitals in the f sub-level
7
max occupancy of the s-sublevel
2 e-
max occupancy of the p sub-level
6 e-
max occupancy of the d sub-level
10 e-
max occupancy of the f-sublevel
14 e-