Molarity Quiz
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
What is molarity?
Amount of dissolved substance contained in a given amount of solution
What is the formula for molarity?
M = liters of solution/moles of solute
How do you find the moles for molarity? (Rearranging)
moles = M × volume (L)
Molarity formula rearrange to volume
volume = M/moles
Solute
The substance that gets dissolved
(eg. Sugar in coffee)
Solution
The substance that dissolves the solute
(eg. The water that dissolves the salt)
What is dilution?
Volume of water that is being added to reduce its concentration.
Formula for dilution
Initial Volume (Vᵢ) x Initial molarity (Mᵢ) = Final Volume (Vf) x Final molarity (Mf)
What doesn’t change in dilution?
Number of moles doesn’t change
Solutions
Using balanced equations to predict how much volume of the standard solutions will be needed to react.
What ways you can show work of how many moles in you solution questions?
Ratio
Equation (to cancel out)
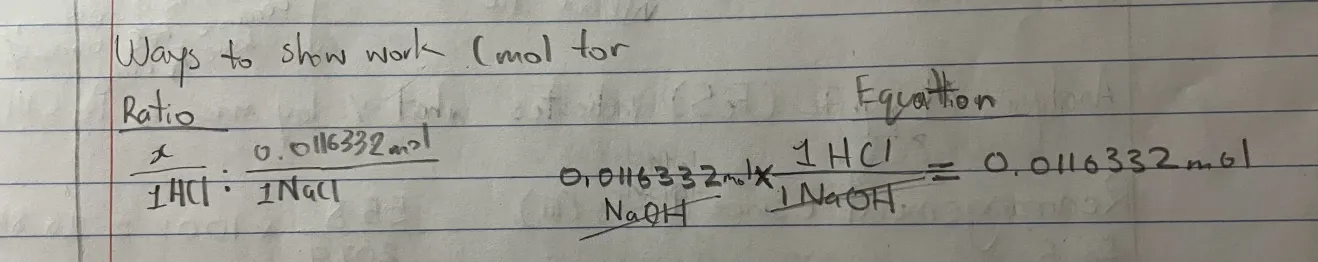
Question: