IB chemistry: topic 16: rate equations + Arrhenius
1/31
Earn XP
Description and Tags
save my exams save my exams pls
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
When the order of reaction with respect to a chemical is 2…
The rate is directly proportional to the square of the concentration of that chemical, e.g. doubling the concentration of the chemical increases the rate of reaction by a factor of four.
The chemical is included in the rate equation (appearing as a squared term).
When the order of reaction with respect to a chemical is 1…
The concentration of the chemical is directly proportional to the rate of reaction, e.g. doubling the concentration of the chemical doubles the rate of reaction.
The chemical is included in the rate equation.
When the order of reaction with respect to a chemical is 0…
Changing the concentration of the chemical has no effect on the rate of the reaction.
Therefore, it is not included in the rate equation.
what do orders that are a fraction suggest?
Orders that are a fraction suggest that the reaction involves multiple steps
what is the order of a reactant?
shows how the concentration of a chemical, typically a reactant, affects the rate of reaction
what can and can’t be featured in a rate equation, besides concentration and order?
Products and catalysts may feature in rate equations.
Intermediates do not feature in rate equations.
are products or reactants used for rate equations?
reactants are used, to the power of their order
what is overall order
all the orders in a rate equation added together
how is the rate equation written?
rate = k (a)^x (b)^y
what is the relationship between rate constant and temperature?
rate constant and temperature have an exponential relationship
zero-order concentration-time graph
the concentration of the reactant is inversely proportional to time.
this means that the reactant concentration decreases as time increases.
the graph is a straight line going down.
rate = k

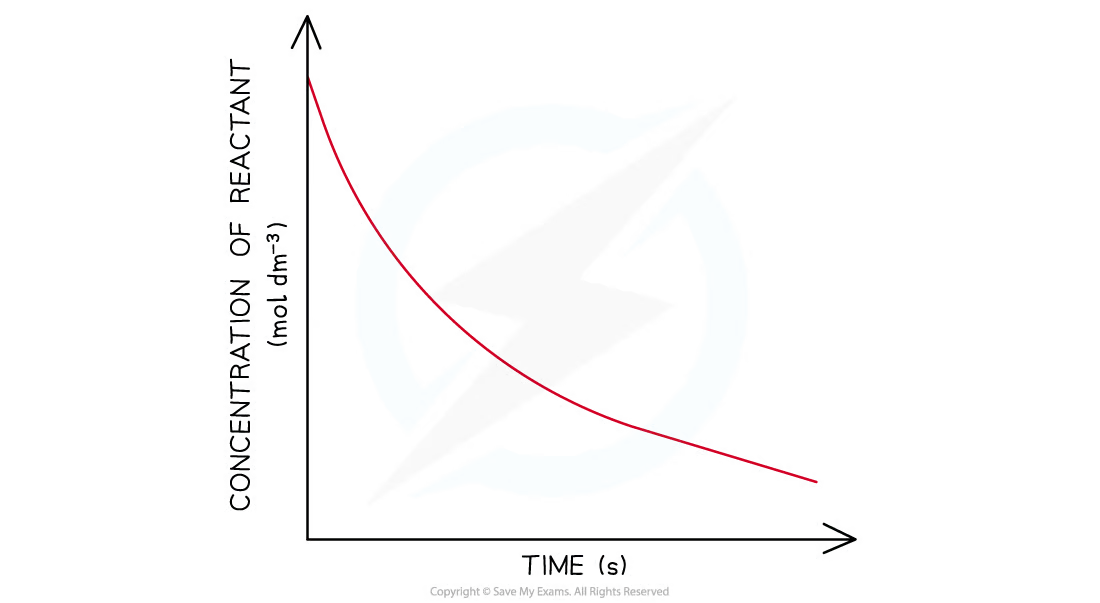
first-order concentration-time graph
the concentration of the reactant decreases with time.
the graph is a curve going downwards and eventually plateaus.
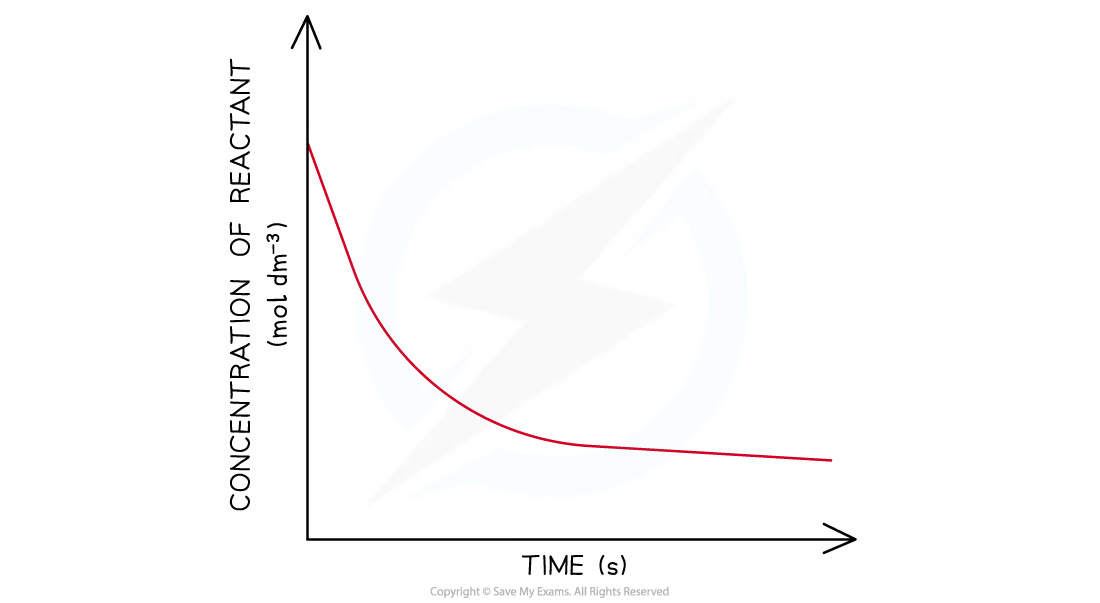
second-order concentration-time graph
the concentration of the reactant decreases more steeply with time.
the concentration of reactant decreases more with increasing time compared to a first-order reaction.
the graph is a steeper curve going downwards.
half life of zero order reactions
for a zero-order reaction the successive half-lives decrease with time
this means that it would take less time for the concentration of reactant to halve as the reaction progresses.
half life of first order reactions
the half-life of a first-order reaction remains constant throughout the reaction.
the amount of time required for the concentration of reactants to halve will be the same during the entire reaction.
half life of second order reactions
for a second-order reaction, the half-life increases with time.
this means that as the reaction is taking place, it takes more time for the concentration of reactants to halve.
zero order rate concentration graph
in a zero-order reaction, the rate doesn’t depend on the concentration of the reactant.
the rate of the reaction therefore remains constant throughout the reaction.
the graph is a horizontal line.
the rate equation is rate = k.

first order rate concentration graph
in a first-order reaction, the rate is directly proportional to the concentration of a reactant.
the rate of the reaction increases as the concentration of the reactant increases.
this means that the rate of the reaction decreases as the concentration of the reactant decreases when it gets used up during the reaction.
the graph is a straight line.
the rate equation is rate = k[A].
![<p>in a first-order reaction, the rate is directly proportional to the concentration of a reactant.</p><p>the rate of the reaction increases as the concentration of the reactant increases.</p><p>this means that the rate of the reaction decreases as the concentration of the reactant decreases when it gets used up during the reaction.</p><p>the graph is a straight line.</p><p>the rate equation is rate = k[A].</p>](https://knowt-user-attachments.s3.amazonaws.com/c41adfcb-a30b-4c47-81fb-ee7f88838f14.png)
second order rate concentration graph
in a second-order reaction, the rate is directly proportional to the square of concentration of a reactant.
the rate of the reaction increases more as the concentration of the reactant increases.
this means that the rate of the reaction decreases more as the concentration of the reactant decreases when it gets used up during the reaction.
the graph is a curved line.
the rate equation is rate = k[A]².
![<p>in a second-order reaction, the rate is directly proportional to the <strong>square</strong> of concentration of a reactant.</p><p>the rate of the reaction increases more as the concentration of the reactant increases.</p><p>this means that the rate of the reaction decreases more as the concentration of the reactant decreases when it gets used up during the reaction.</p><p>the graph is a curved line.</p><p>the rate equation is rate = k[A]².</p>](https://knowt-user-attachments.s3.amazonaws.com/d4b0ea6a-af07-4059-afcf-688752ce64e0.png)
what is the rate determining step
the slowest step in the reaction:
a chemical reaction can only go as fast as the slowest part of the reaction.
what is the relationship between rate equation and rate determining step
If a reactant appears in the rate-determining step, then the concentration of that reactant will also appear in the rate equation.
what is molecularity?
the number of reactant particles that participate in the rate-determining step
what does unimolecular mean?
one species involved in the rate-determining step
what does bimolecular mean?
two species involved in the rate-determining step
what is an intermediate?
the product of the reactants in the rate-determining step.
what is the transition state?
high energy transition between the forming of products and collision of reactants
in an energy profile, it is the hump at the top of the activation energy.

how does the activation energy relate to the rate determining step?
The reaction step with the greatest activation energy will be the rate-determining step and can be used to determine the rate equation.
what factors affect k?
temperature ONLY
how is rate/rate constant affected by temperature?
as temperature increases, more kinetic energy, more frequent successful collisions,faster rate of reaction THEREFORE higher rate constant.
rate and rate constant are directly proportional.
what is arrhenius equation? (without ln)
K = A e ^-Ea/RT
K = rate constant . A = arrhenius constant . Ea = activation energy . R = gas constant . T = temperature.

what is arrhenius equation? (with ln)
lnK = lnA -Ea/RT
K = rate constant . A = arrhenius constant . Ea = activation energy . R = gas constant . T = temperature.
what is the relationship between arrhenius plots and activation energy?
the reaction plotted with a steeper gradient has the higher activation energy, Ea.
this indicates that the rate constant, and therefore rate, will change quicker with temperature changes.
