Dynamic equilibria + Le Chatelier's principle
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
What is dynamic equilibrium?
When the rate of the forwards and backwards reaction is equal in a reversible reaction
When cna dynamic equilibrium occur?
In a closed system
What holds true in a dynamic equilibrium?
Concentration of reactants and products remain constant
What is le Chatelier’s principle?
Le Chatelier's Principle- If a reaction in equilibrium is subjected to a change in pressure, temperature or concentration, the position of equilibrium will move to counteract the change
What are factors that affect Le Chatelier’s principle?
Concentration of reactants
Pressure
Temperature
Catalyst
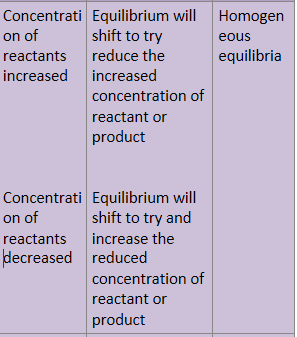
How does concentration effect equilibrium and which equlibria will be affected?
How does pressure affect equilibrium and which equilibria will be affected?

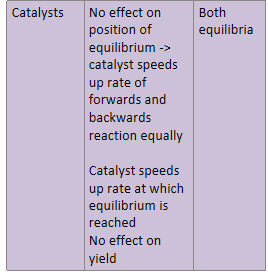
How do catalysts affect equilibrium and which equilibria will be affected?