AP Bio Final
1/46
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
47 Terms
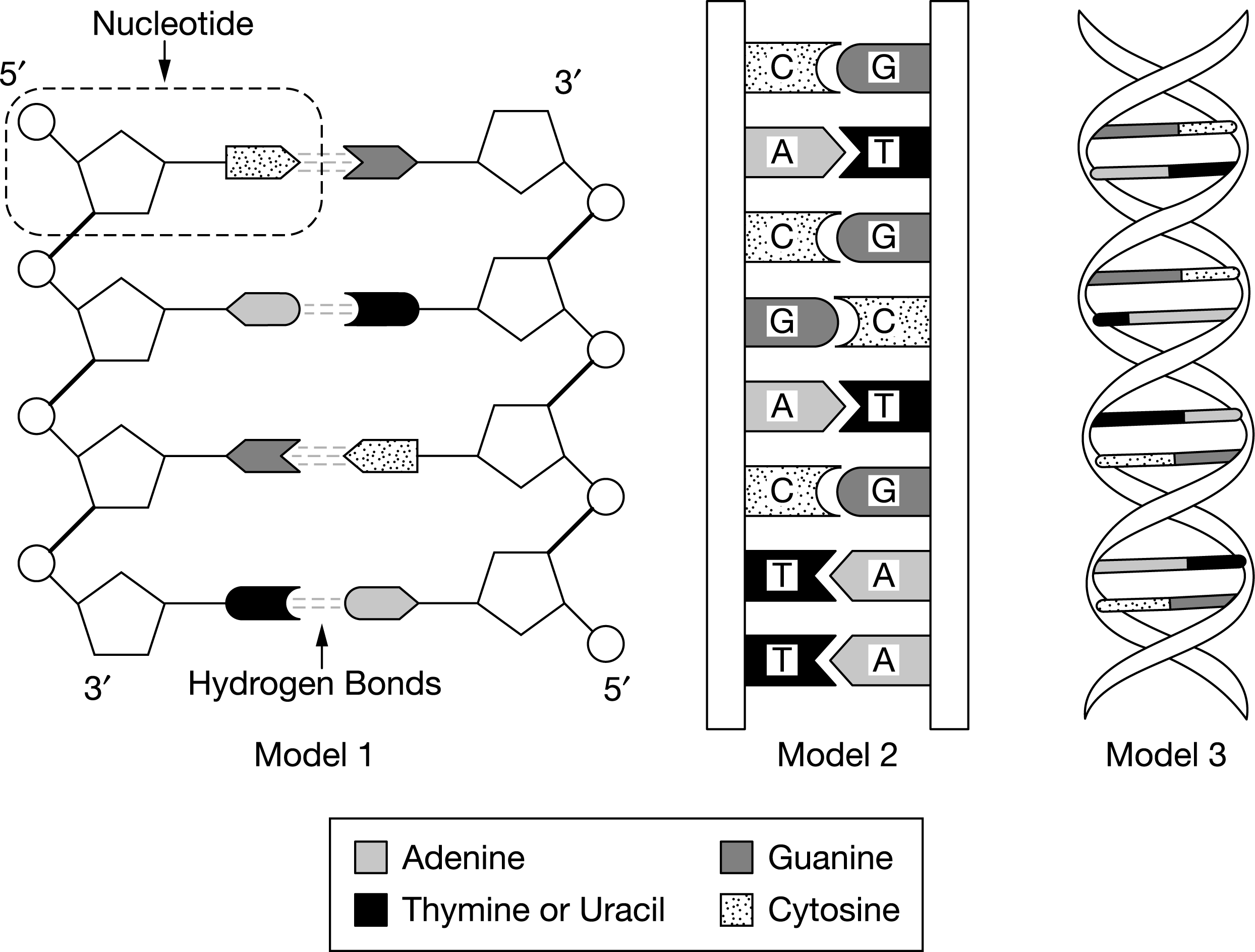
Which feature of model 1 best illustrates how biological information is coded in a DNA molecule?
a. The 5′ and 3′ labels at the ends of each strand
b. The labeling of the hydrogen bonds between base pairs
c. The lines connecting sugars and phosphate groups that represent covalent bonds
d. The linear sequence of the base pairs
d. The linear sequence of the base pairs
The sequence of base pairs in a DNA molecule plays a central role in the coding of biological information (Unit 1).
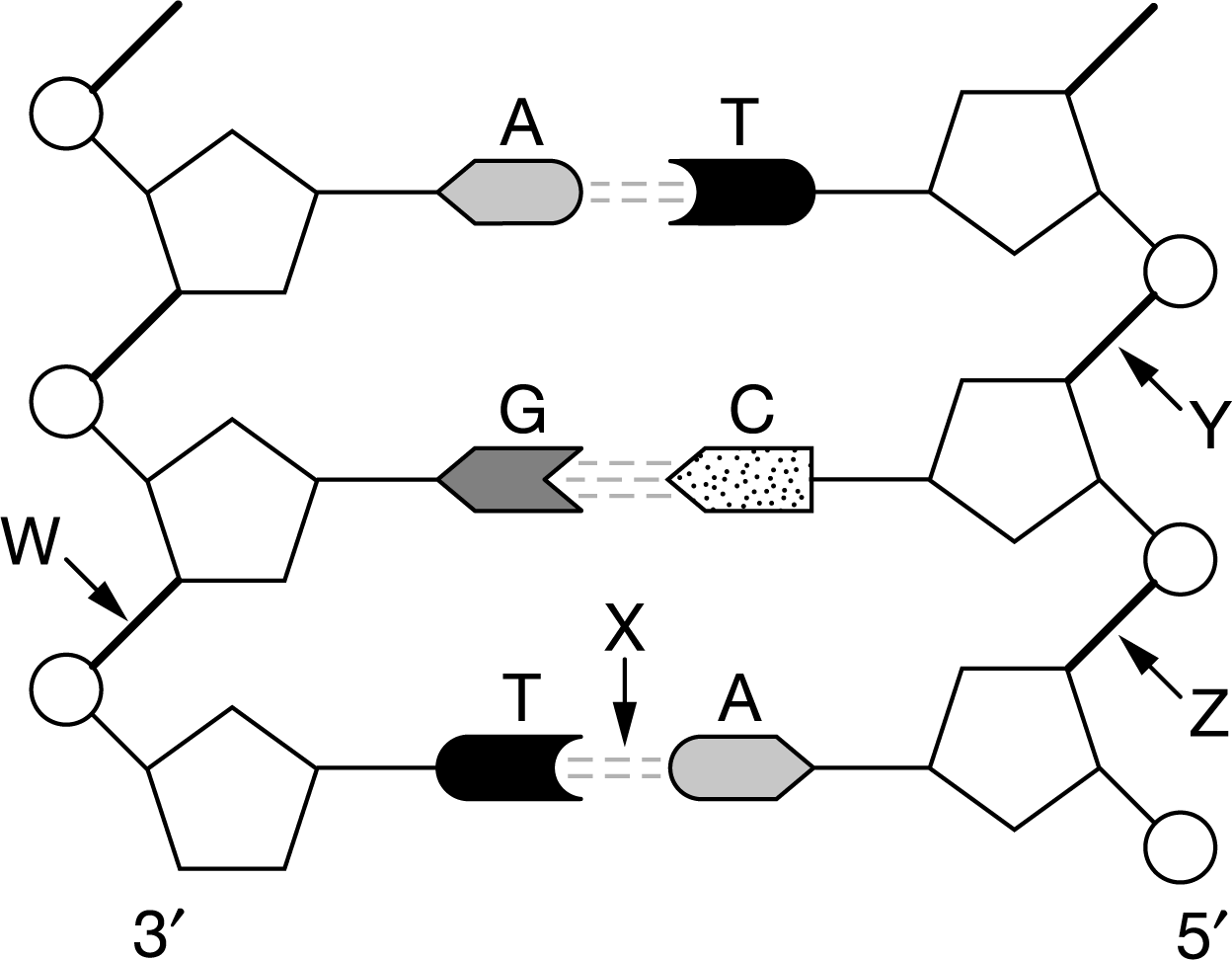
Figure 1 represents a segment of DNA. Radiation can damage the nucleotides in a DNA molecule. To repair some types of damage, a single nucleotide can be removed from a DNA molecule and replaced with an undamaged nucleotide. Which of the four labeled bonds in Figure 1 could be broken to remove and replace the cytosine nucleotide without affecting the biological information coded in the DNA molecule?
a. Bond X only
b. Bond W only
c. Bonds Y and Z at the same time
d. Bonds W and Z at the same time
c. Bonds Y and Z at the same time
By breaking bonds Y and Z at the same time, the cytosine nucleotide could be removed from the DNA molecule and replaced with an undamaged cytosine without changing the biological information stored in the DNA (Unit 1).
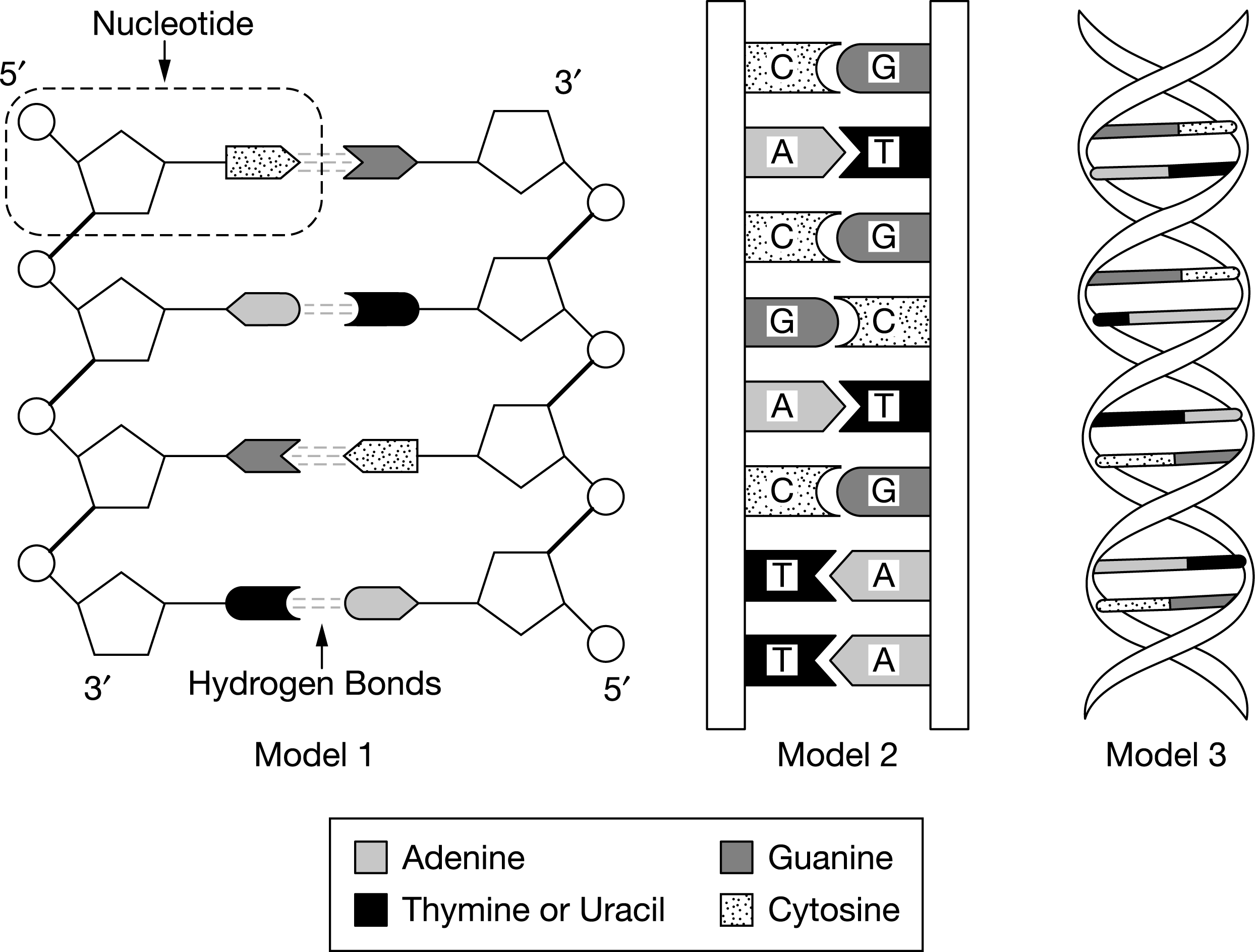
A student wants to modify model 1 so that it represents an RNA double helix instead of a DNA double helix. Of the following possible changes, which would be most effective in making model 1 look more like RNA than DNA?
a. Changing the sequence of the base pairs
b. Changing the deoxyriboses to riboses by adding −OH groups
c. Changing the shapes of the nitrogenous bases to match those shown in model 2
d. Changing the sugar-phosphate backbone to a ribbon, as shown in model 3
b. Changing the deoxyriboses to riboses by adding −OH groups
RNA contains ribose, whereas DNA contains deoxyribose. A ribose sugar has an −OH group linked to the 2′ carbon that a deoxyribose sugar does not have (Unit 1).
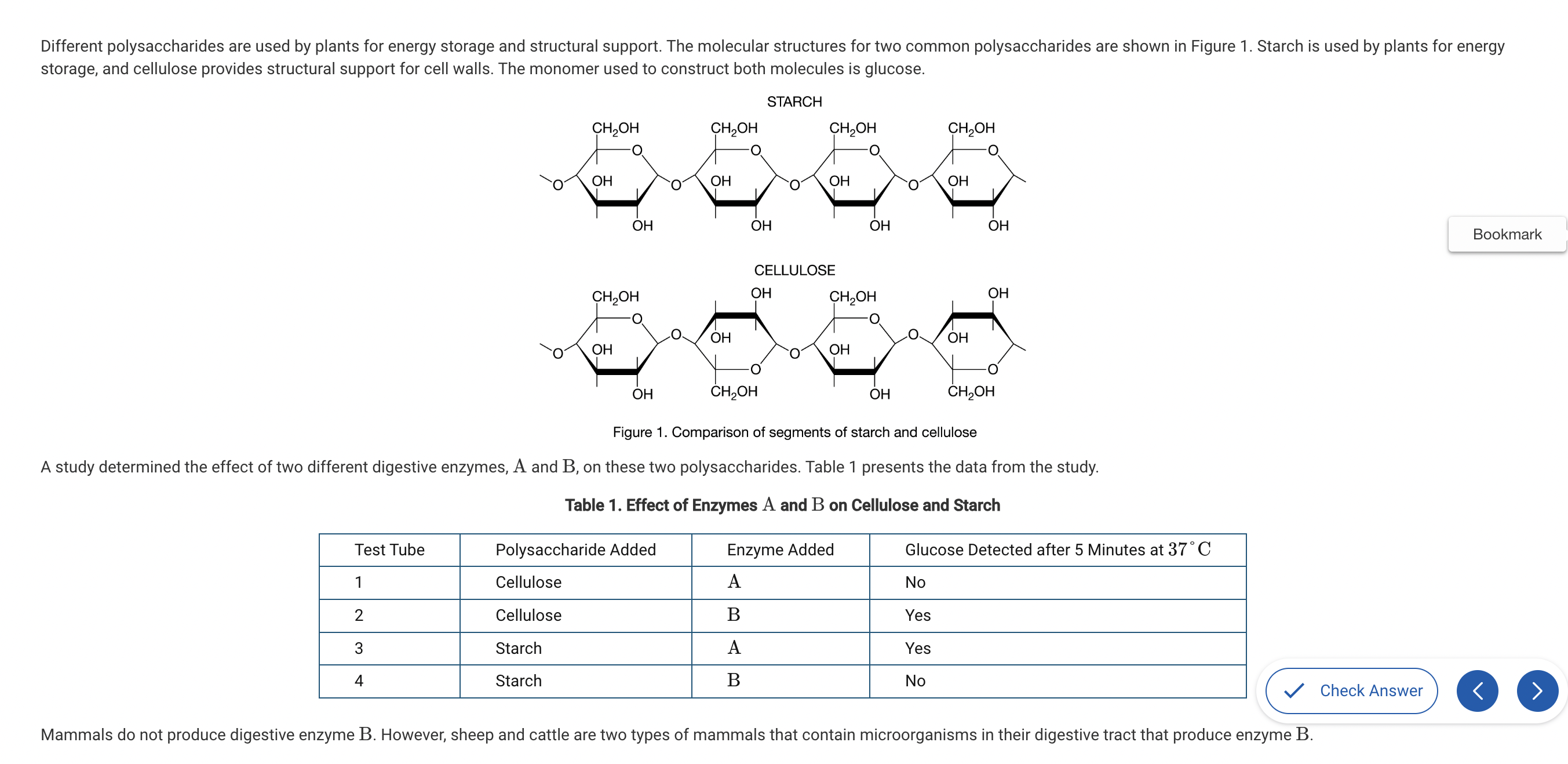
Based on Figure 1, which of the following best compares the atomic structures of starch and cellulose?
a. Starch is composed of carbon, hydrogen, and oxygen, while cellulose also contains nitrogen.
b. Starch and cellulose are composed of repeating glucose monomers; however, in cellulose every other glucose monomer is rotated 180 degrees.
c. Starch is composed of monomers that each have a CH2OH group, while cellulose only has a CH2OH group on every other monomer.
d. Starch and cellulose are composed of identical monomers and therefore have identical structures.
b. Starch and cellulose are composed of repeating glucose monomers; however, in cellulose every other glucose monomer is rotated 180 degrees.
Both starch and cellulose are both composed of repeating glucose molecules; however, the orientation of every other glucose in cellulose is upside down compared with the ones on either side (Unit 1).
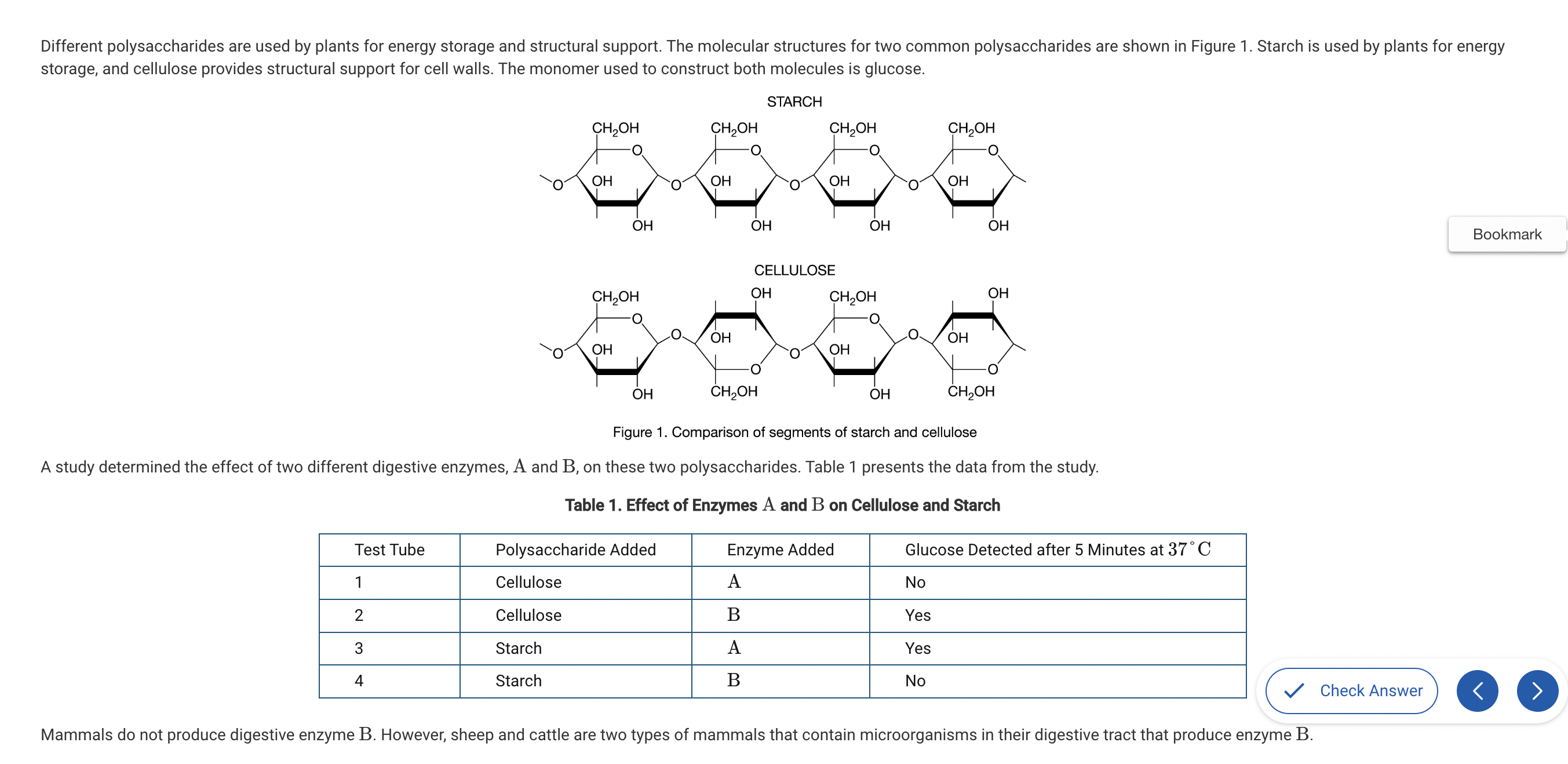
Which of the following statements best describes the different functions of starch and cellulose in plants?
a. The differences in the assembly and organization of the monomers of these two polymers result in different chemical properties.
b. Since starch and cellulose are composed of identical monomers, the cellular environment where they are located controls their function..
c. The monomers of cellulose are connected by covalent bonds, making it idea for structural support.
d. The monomers of starch are connected by ionic bonds, making it ideal for energy storage for plants.
a. The differences in the assembly and organization of the monomers of these two polymers result in different chemical properties.
The identical orientations of the glucose monomers in starch create a polysaccharide with alpha bonds that is easy to break down into glucose for energy use. The alternating orientations of the glucose monomers in cellulose create beta bonds that produce a rigid polymer that is difficult to digest for energy use (Unit 1).
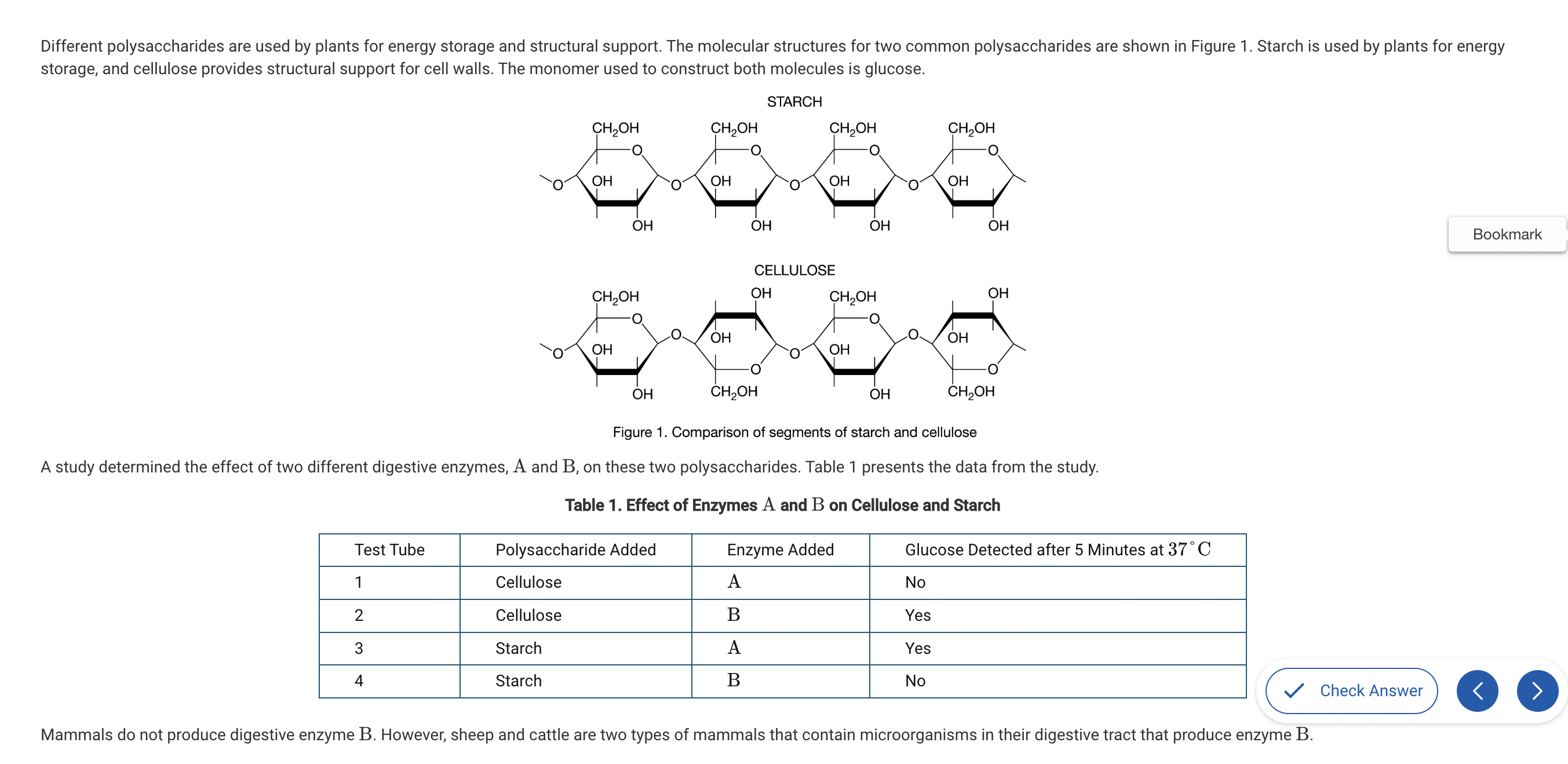
Which of the following best describes the process that adds a monosaccharide to an existing polysaccharide?
a. The monosaccharide is completely broken down by a specific enzyme and then the atoms are reorganized and made into a polysaccharide.
b. Ionic bonds are formed between adjacent carbon atoms of the monosaccharide and the polysaccharide by adding water (H2O) and a specific enzyme.
c. A specific enzyme removes the hydrogen (H) from the monosaccharide and the hydroxide (OH) from the polysaccharide, creating a bond between the two and creating a water (H2O) molecule.
d. A specific enzyme removes two hydroxides (OH), one from the monosaccharide, and one from the polysaccharide, creating a bond between the two monosaccharides and creating a hydrogen peroxide (H2O2) molecule.
c. A specific enzyme removes the hydrogen (H) from the monosaccharide and the hydroxide (OH) from the polysaccharide, creating a bond between the two and creating a water (H2O) molecule.
This is a description of dehydration synthesis, which joins multiple monosaccharides to create a polysaccharide and produces water (H2O) molecules (Unit 1).
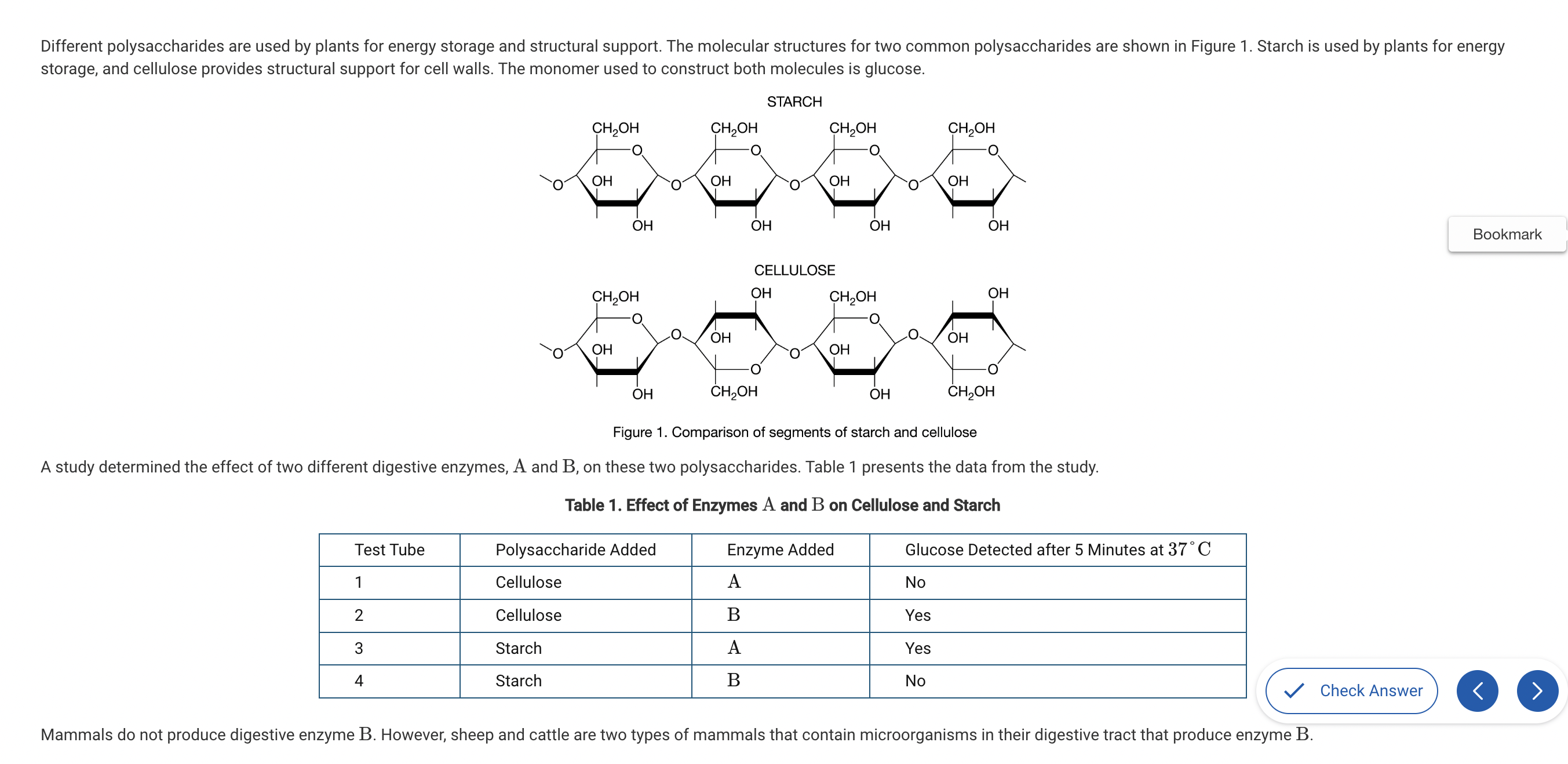
Which of the following would most likely occur if cattle lost the ability to maintain a colony of microorganisms in their digestive tract?
a. Cattle would no longer be able to synthesize cellulose.
b. Cattle would have to convert cellulose to starch before digesting it.
c. Cattle would have to start producing enzyme 𝐵 without the help of the bacteria.
d. Cattle would no longer be able to use cellulose as a primary source of glucose.
d. Cattle would no longer be able to use cellulose as a primary source of glucose.
Without the enzyme 𝐵 produced by microorganisms in their digestive tract, cellulose would pass through the digestive tract without being digested (Unit 1).
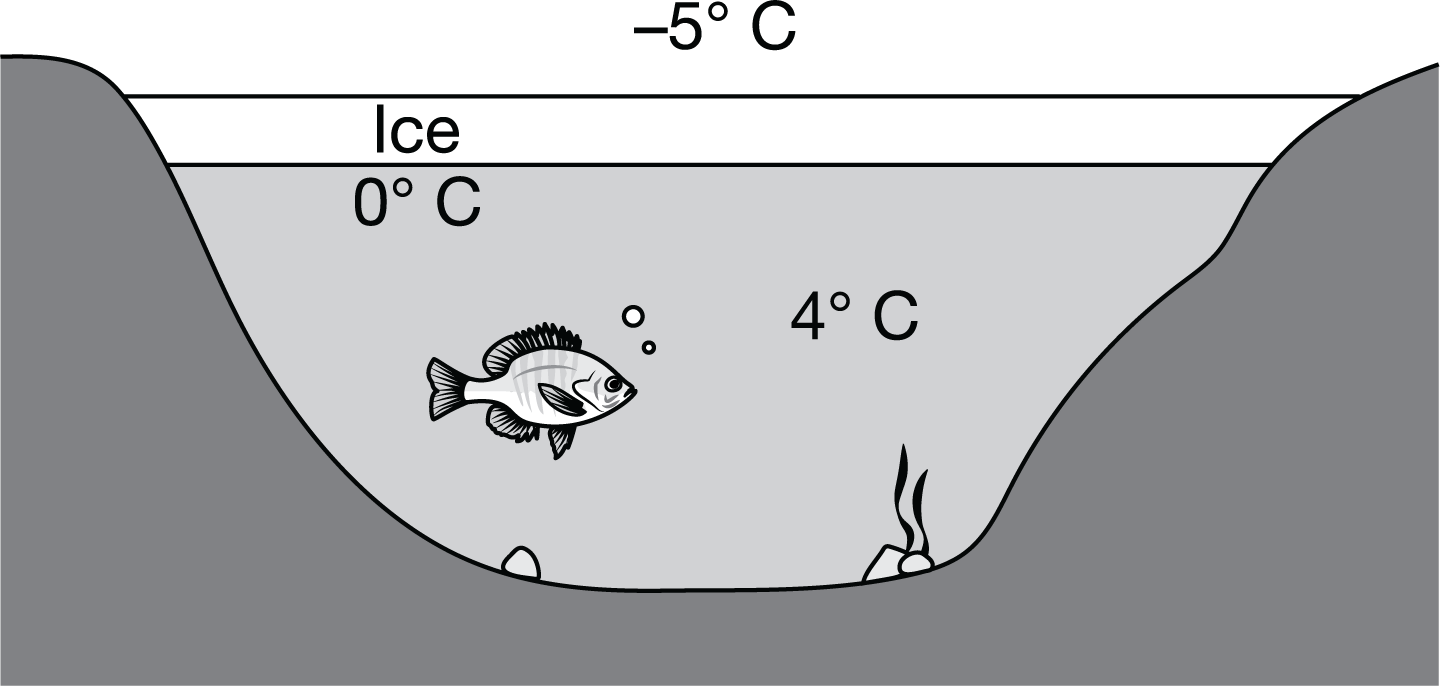
As shown in the diagram, when environmental temperatures drop below freezing, a layer of ice typically forms on the surface of bodies of freshwater such as lakes and rivers. Which of the following best describes how the structure of ice benefits the organisms that live in the water below?
a. The water molecules in ice are closer together than those in liquid water, so the ice prevents the passage of air to the water, maintaining a constant gas mixture in the water.
b. The water molecules in ice are closer together than those in liquid water, so the ice forms a barrier that protects the organisms in the water from the freezing air temperatures.
c. The water molecules in ice are farther apart than those in liquid water, so the ice floats, maintaining the warmer, denser water at the lake bottom.
d. The water molecules in ice are farther apart than those in liquid water, so the ice floats, preventing the escape of gases from the liquid water.
c. The water molecules in ice are farther apart than those in liquid water, so the ice floats, maintaining the warmer, denser water at the lake bottom.
The water molecules in ice are farther from each other than are water molecules in liquid water, so ice is less dense than liquid water and floats on its surface, while the denser water at 4 degrees Celsius sinks to the bottom, maintaining a steady temperature all winter (Unit 1).
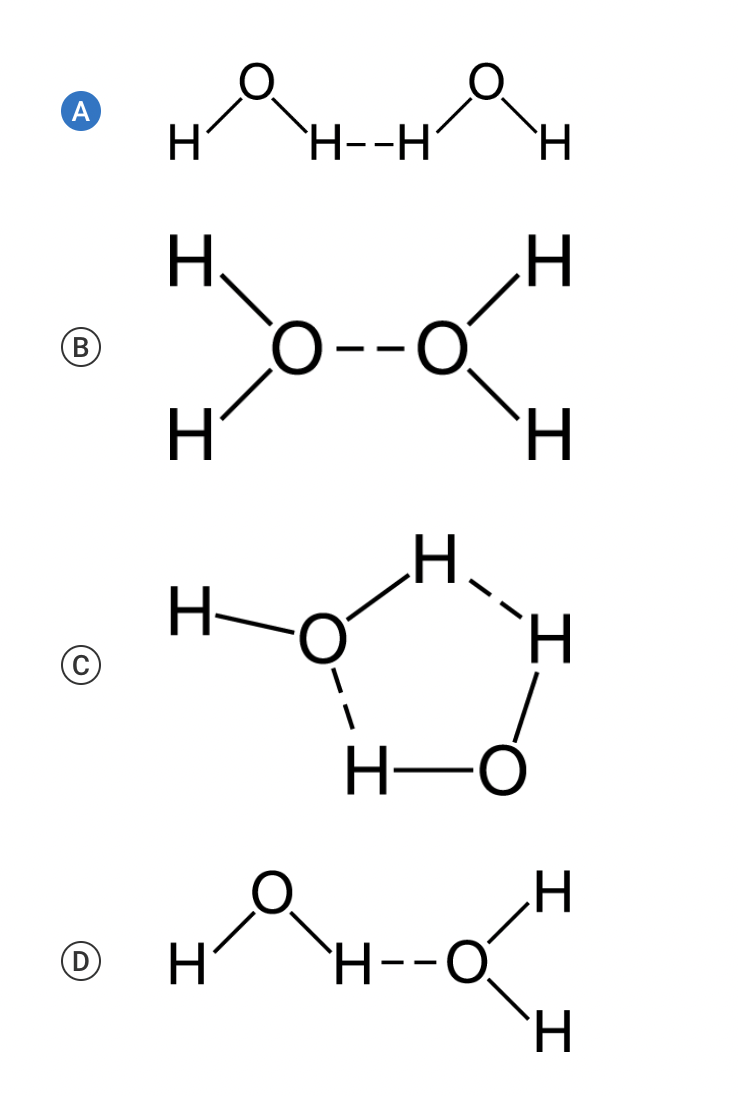
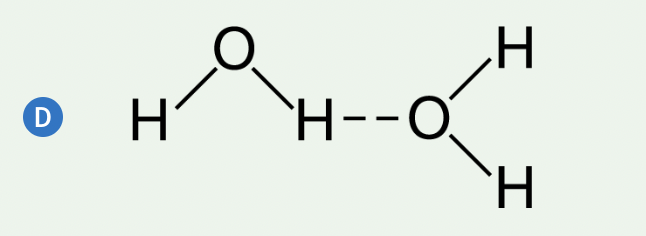
Water molecules are polar covalent molecules. There is a partial negative charge near the oxygen atom and partial positive charges near the hydrogen atoms due to the uneven distribution of electrons between the atoms, which results in the formation of hydrogen bonds between water molecules. The polarity of water molecules contributes to many properties of water that are important for biological processes. Which of the following models best demonstrates the arrangement of hydrogen bonds between adjacent water molecules?
a, b, c, or d
The hydrogen bonds between these water molecules correctly show the attractive force between the hydrogen atom of one water molecule and the oxygen atom of the adjacent water molecule (Unit 1).
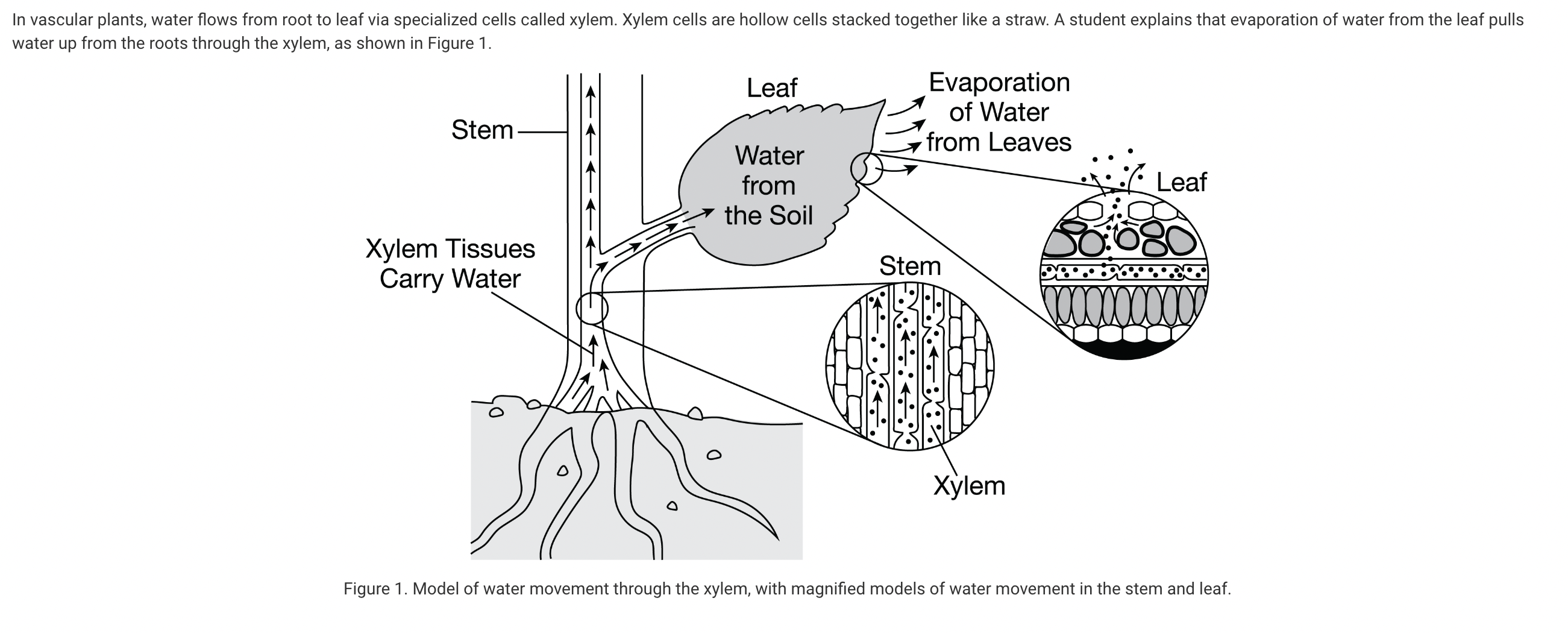
Which statement describes how water is pulled up through the xylem to the leaves of the plant?
a. As water exits the leaf, hydrogen bonding between water molecules pulls more water up from below.
b. As water exits the leaf, signals are sent to the roots to pump more water up to the leaves through the xylem by adhesion.
c. Evaporation from the leaf decreases the hydrogen bonds that form between the water molecules in the xylem, which helps the water molecules to be pulled up the xylem.
d. Evaporation of water from the leaf increases the hydrogen bonds that form between water molecules in the air, providing the energy for transport.
a. As water exits the leaf, hydrogen bonding between water molecules pulls more water up from below.
As water exits the leaf, hydrogen bonding pulls more water molecules up through the leaf and xylem by cohesion (Unit 1).
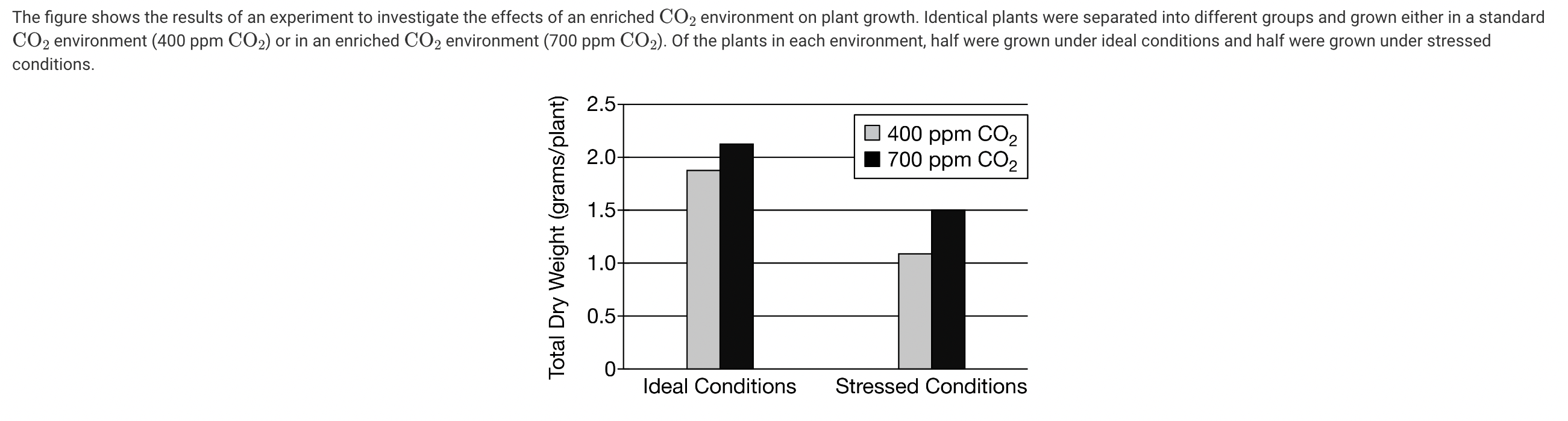
Based on the figure, which statement best describes the observed relationship between atmospheric CO2 enrichment and plant growth under ideal and stressed conditions?
a. The increase in atmospheric CO2 had no observable effect on plant growth under either ideal or stressed conditions.
b. The increase in atmospheric CO2 resulted in a greater increase in plant growth under ideal conditions than under stressed conditions.
c. The increase in atmospheric CO2 resulted in a greater increase in plant growth under stressed conditions than under ideal conditions.
d. The increase in atmospheric CO2 resulted in an inhibition of plant growth under both ideal and stressed conditions.
c. The increase in atmospheric CO2 resulted in a greater increase in plant growth under stressed conditions than under ideal conditions.
Based on the figure, the increase in atmospheric CO2 resulted in a greater increase in plant growth under stressed conditions than under ideal conditions (Unit 1).
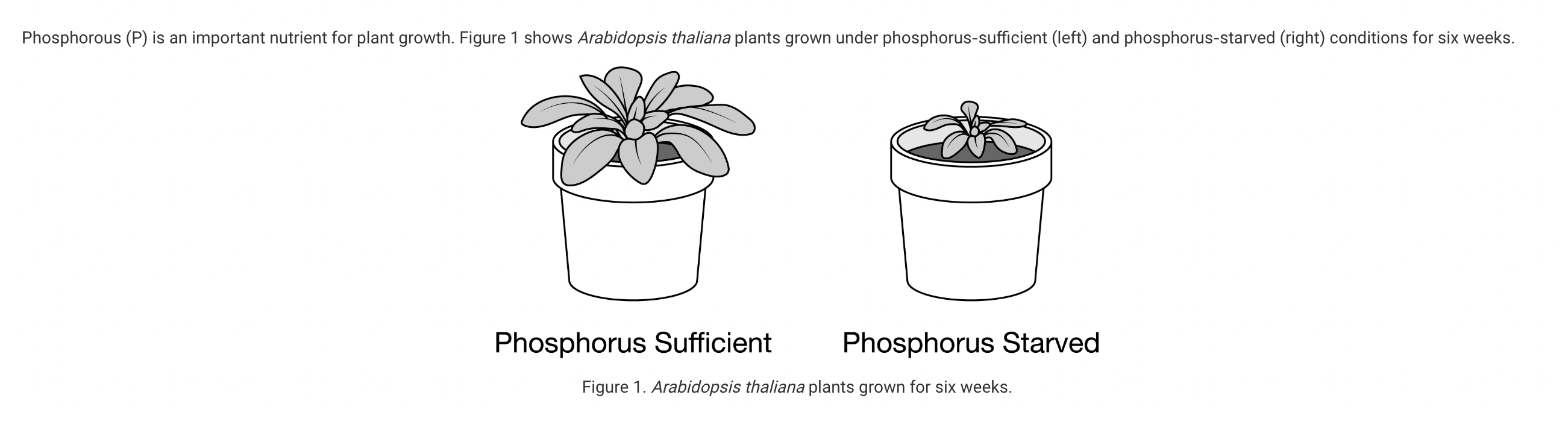
Which of the following is the most likely reason for the difference in leaf growth?
a. The phosphorus-starved plant was unable to synthesize both the required proteins and lipids, limiting growth.
b. The phosphorus-starved plant was unable to synthesize both the required proteins and carbohydrates, limiting growth.
c. The phosphorus-starved plant was unable to synthesize both the required nucleic acids and lipids, limiting growth.
d. The phosphorus-starved plant was unable to synthesize both the required carbohydrates and nucleic acids, limiting growth.
c. The phosphorus-starved plant was unable to synthesize both the required nucleic acids and lipids, limiting growth.
Phosphorus is used to make nucleic acids and certain lipids. Without phosphorus atoms, nucleic acids and lipids cannot be made for the plant to use for growth (Unit 1).
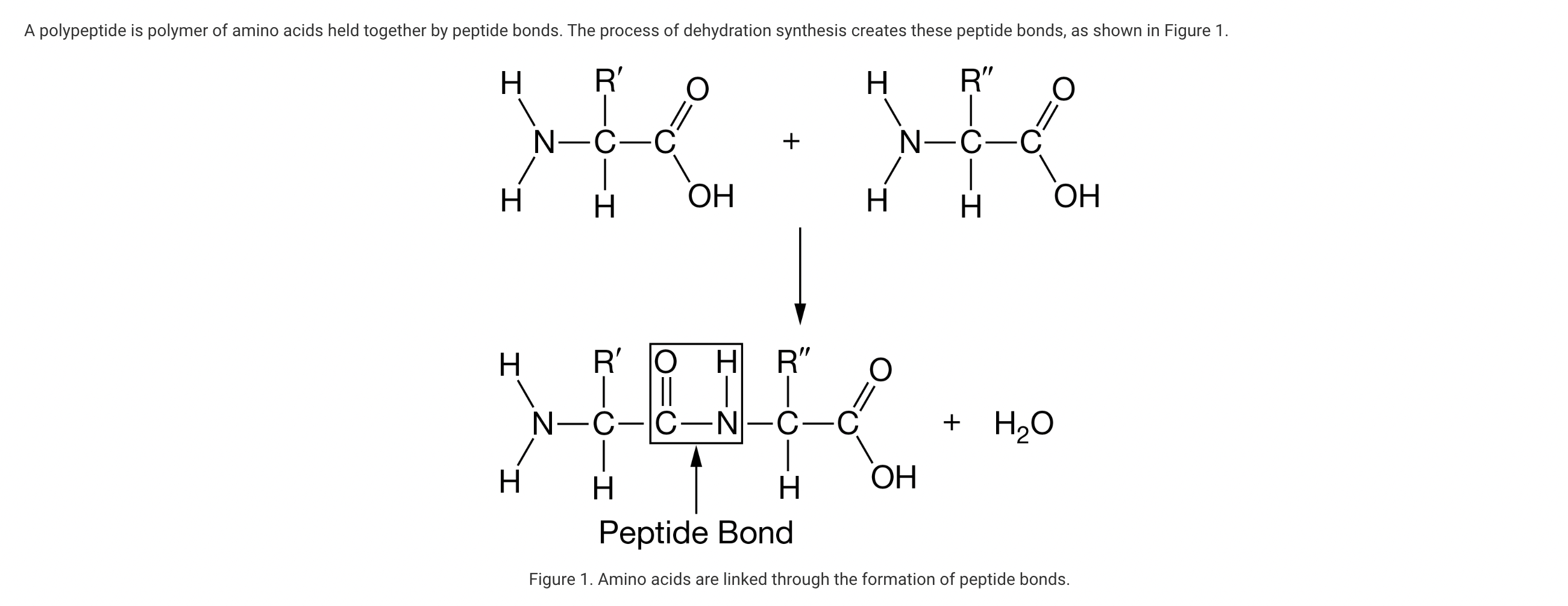
As shown in Figure 1, an amino acid must have which of the following properties in order to be incorporated into a polypeptide?
a. The ability to remain stable in the presence of water molecules
b. An R-group that is compatible with the R-group of the last amino acid incorporated
c. A central carbon atom that reacts with a nitrogen atom to form the peptide bond
d. The ability to form a covalent bond with both its NH2 group and its COOH group
d. The ability to form a covalent bond with both its NH2 group and its COOH group
An amino acid needs to participate in the formation of two peptide bonds in order to be part of a polypeptide. The first bond may occur when the NH2 group interacts with the COOH group of another amino acid. The second bond occurs when the COOH group interacts with the NH2 group of a third amino acid (Unit 1).
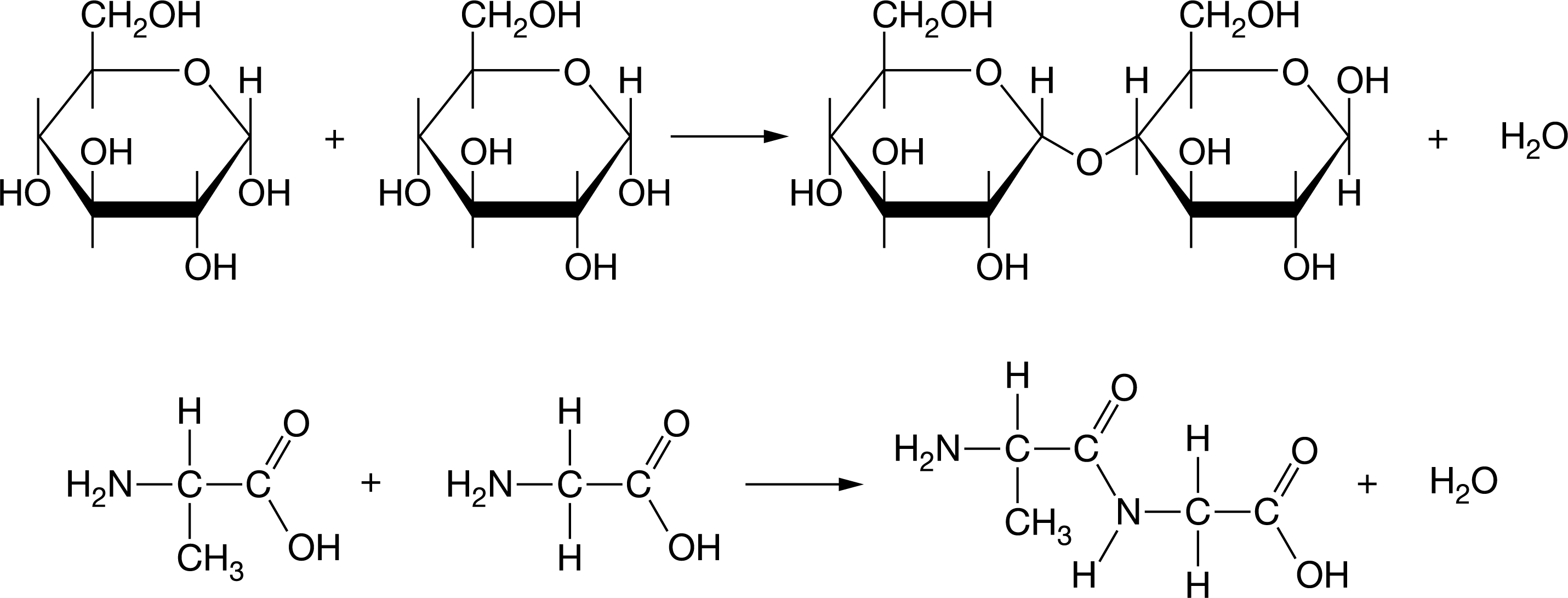
Which of the following is common feature of the illustrated reactions showing the linking of monomers to form macromolecules?
a. Two identical monomers are joined by a covalent bond.
b. Two different monomers are joined by a covalent bond.
c. Monomers are joined by a covalent bond, and a water molecule is produced.
d. Monomers are joined by ionic bonds, and a water molecule is produced.
c. Monomers are joined by a covalent bond, and a water molecule is produced.
The monomers of the two reactions illustrated are joined by covalent bonds with the production of a water molecule (Unit 1).
Which of the following describes a key difference among the 20 amino acids that are used to make proteins?
a. Only some amino acids have an R-group.
b. Only some amino acids have a carboxyl group (COOH).
c. Some amino acids are hydrophobic.
d. Some amino acids contain the element phosphorus.
c. Some amino acids are hydrophobic.
Due to having nonpolar R-groups, 10 of the 20 amino acids are hydrophobic. Interactions between hydrophobic amino acids play an important role in determining protein structure and function (Unit 1).
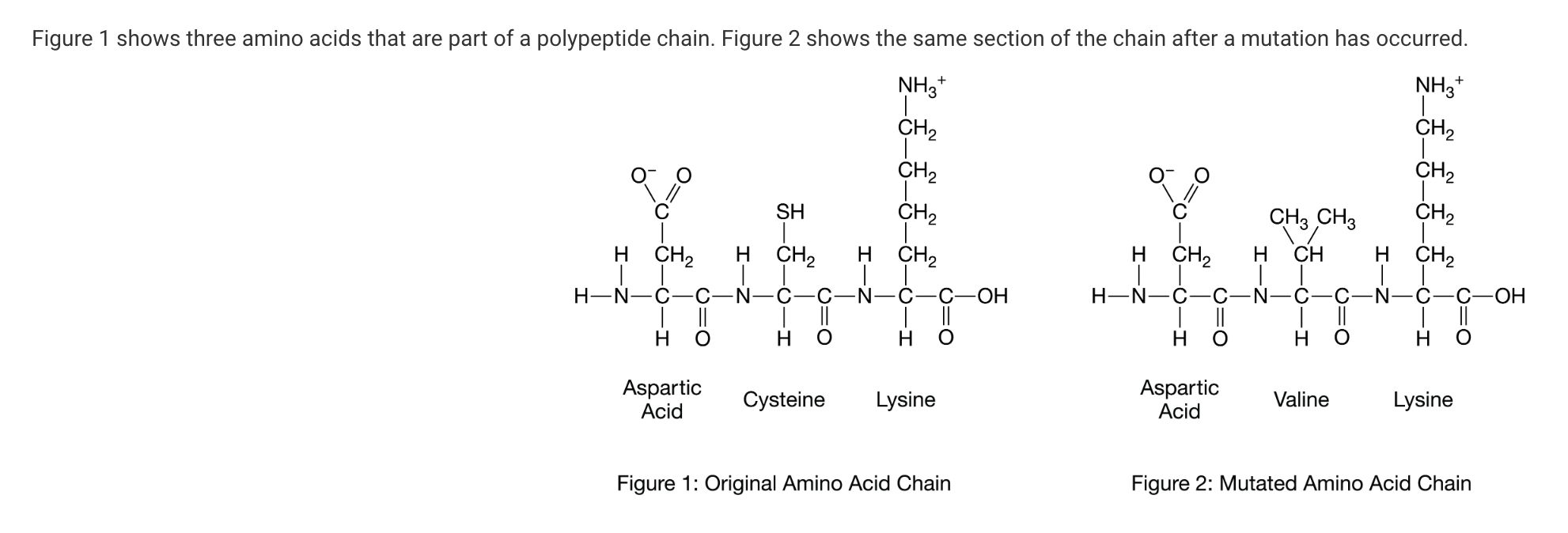
How might this change affect the structure and function of the protein?
a. The R-group of the new amino acid, valine, has different chemical properties than the R-group of cysteine. This will cause the protein to misfold and not function properly in the cell.
b. The new amino acid, valine, has replaced cysteine in the new protein. Since the number of amino acids has remained the same, there will be no change in the three-dimensional folding, or function, of the protein.
c. Since this is a linear section, it does not influence protein folding. Thus, there will be no change in protein structure or function.
d. Since the new amino acid is bounded on one side by an amino acid with a negatively charged R-group and by an amino acid on the other side with a positively charged R-group, the charges will balance and the protein will fold as usual.
a. The R-group of the new amino acid, valine, has different chemical properties than the R-group of cysteine. This will cause the protein to misfold and not function properly in the cell.
Three-dimensional folding of a protein is due to interactions among the R groups of the amino acids. Cysteine has a sulfhydryl group, which may form a disulfide bridge with another part of the polypeptide chain. Valine has no sulfhydryl group and is nonpolar, which will affect how the polypeptide will fold. (Unit 1).
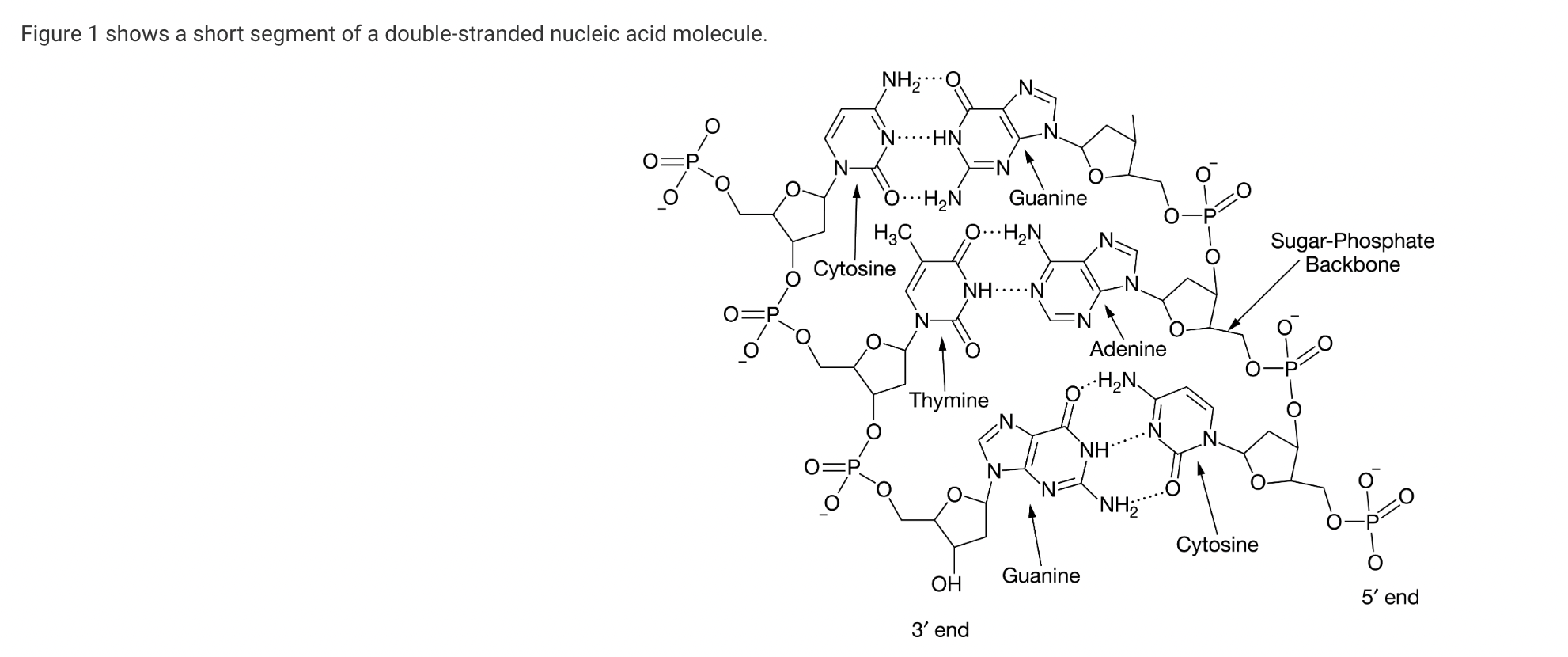
Which of the following statements is correct about the molecule shown in Figure 1 ?
a. It is RNA because of the relative direction of the two strands.
b. It is RNA because of the number of different nucleotides found in the molecule.
c. It is DNA because of the nature of the hydrogen bonds between guanine and cytosine.
d. It is DNA because of the nucleotides present.
d. It is DNA because of the nucleotides present.
The figure indicates that one of the nucleotides is thymine. Thymine is found in DNA and not in RNA (Unit 1).
Which of the following best describes a structural similarity between the two molecules shown in Figure 1 that is relevant to their function?
a. Both molecules are composed of the same four nucleotides, which allows each molecule to be produced from the same pool of available nucleotides.
b. Both molecules are composed of the same type of five-carbon sugar, which allows each molecule to act as a building block for the production of polysaccharides.
c. Both molecules contain nucleotides that form base pairs with other nucleotides, which allows each molecule to act as a template in the synthesis of other nucleic acid molecules.
d. Both molecules contain nitrogenous bases and phosphate groups, which allows each molecule to be used as a monomer in the synthesis of proteins and lipids.
c. Both molecules contain nucleotides that form base pairs with other nucleotides, which allows each molecule to act as a template in the synthesis of other nucleic acid molecules.
Nucleotides form base pairs with other nucleotides. The base pairing allows a strand of RNA or DNA to act as a template in the synthesis of other nucleic acid molecules. Examples include the cellular processes of DNA replication (DNA is used as a template to make DNA), transcription (DNA is used as a template to make RNA), and reverse transcription (RNA is used as a template to make DNA) (Unit 1).
A certain type of specialized cell contains an unusually large amount of rough endoplasmic reticulum (ER). Which of the following functions is this cell type most likely specialized to perform?
a. The production and secretion of steroids
b. The destruction of toxic materials produced in other cells of the organism
c. The synthesis of polysaccharides for energy storage
d. The production and secretion of proteins
d. The production and secretion of proteins
The cytosolic surface of the rough ER is covered by ribosomes that synthesize proteins that are then transported into the rough ER, then to the Golgi complex, and finally out of the cell (Unit 2).
Which of the following cellular deficiencies would most likely be related to mutations in mitochondrial proteins?
a. The cell is unable to synthesize most proteins required for normal cell functions.
b. The cell is unable to break down toxic materials and would accumulate large volumes of these materials.
c. The cell is able to synthesize proteins, but the proteins would not fold properly and would not contain the correct molecular tags for export from the cell.
d. The cell is unable to complete reactions related to electron transport and ATP production.
d. The cell is unable to complete reactions related to electron transport and ATP production.
The electron transport chain and ATP production are associated with proteins in the inner membrane of the mitochondrion. Nonfunctional proteins in the mitochondrion are likely to result in reduced ATP production (Unit 2).
A scientist is studying the various prokaryotic and eukaryotic species found floating in a sample of water taken from a marine ecosystem.
Which cellular component will be found in the widest range of organisms in the sample?
a. The chloroplast, since all organisms need a source of energy.
b. The ribosome, since all organisms need to synthesize proteins.
c. The mitochondrion, since all organisms need to break down glucose.
d. The cell wall, since all marine organisms need them for support.
b. The ribosome, since all organisms need to synthesize proteins.
Ribosomes are found in all forms of life, allowing for comparison of the widest possible range of plankton species (Unit 2).
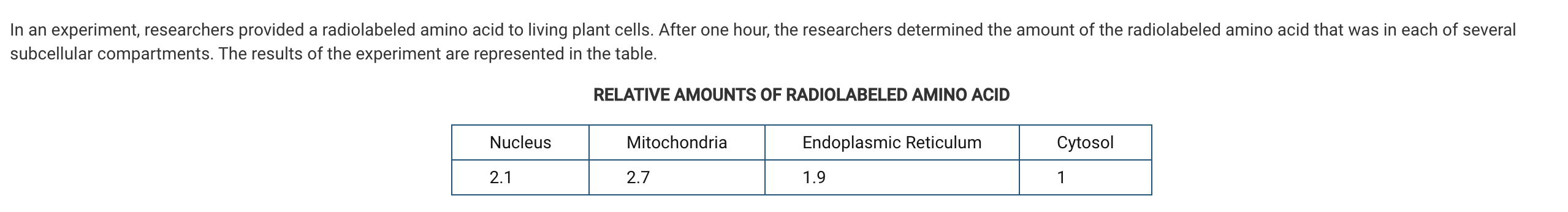
Which of the following conclusions about the radiolabeled amino acid is best supported by the results of the experiment?
a. It was mostly incorporated into nucleic acids that store the biological information.
b. It was mostly incorporated into proteins that regulate and manage metabolic reactions.
c. It was mostly incorporated into lipids that help separate cells from their surrounding environment.
d. It was mostly incorporated into carbohydrates that form protective structures outside the cells.
b. It was mostly incorporated into proteins that regulate and manage metabolic reactions.
Amino acids are the building block of proteins, and the data indicate that most of the radiolabeled amino acid was in the mitochondria. Therefore, the data best support the conclusion that most of the amino acid molecules were incorporated into proteins that regulate and manage the metabolic reactions that occur in mitochondria (Unit 2).
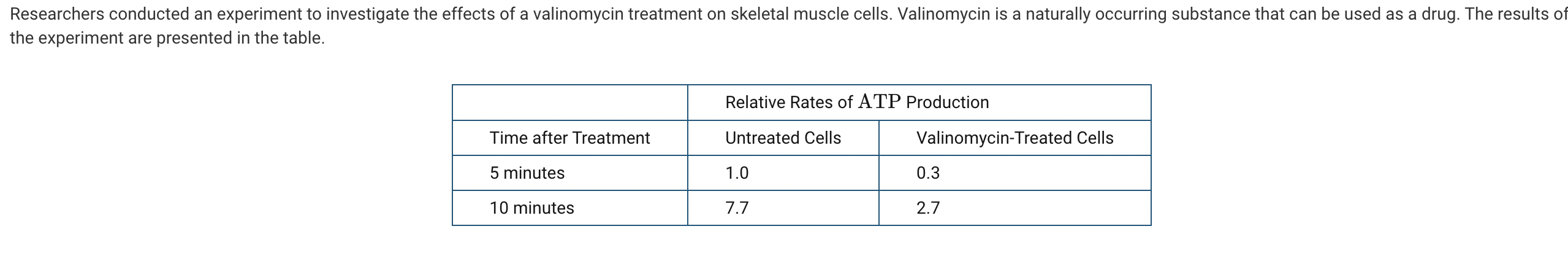
Which of the following claims about the effects of the valinomycin treatment is best supported by the data presented in the table?
a. The valinomycin treatment caused an increase in the activity of the rough endoplasmic reticulum.
b. The valinomycin treatment caused an increase in the activity of the Golgi complex.
c. The valinomycin treatment caused a decrease in the activity of the lysosome.
d. The valinomycin treatment caused a decrease in the activity of the mitochondria.
d. The valinomycin treatment caused a decrease in the activity of the mitochondria.
The data indicate that the valinomycin treatment caused a decrease in the relative rate of ATP production, which likely resulted from impaired mitochondrial function (Unit 2).
In an experiment, researchers compared the growth of two different plants, plant X and plant Y. The researchers maintained the plants under nearly identical conditions and observed that plant X grew faster than plant Y. The researchers also observed that the inner mitochondrial membranes of plant X had more folds than did those of plant Y.
Which of the following conclusions about increasing the number of folds in the inner mitochondrial membrane is best supported by the results of the experiment?
a. It increases the efficiency of photosynthesis, which results in faster cell growth.
b. It increases the surface area available for ATP production, which results in faster cell growth.
c. It increases the amount of space available for storing cellular wastes, which results in faster cell growth.
d. It increases the rate of protein transport to the plasma membrane, which results in faster cell growth.
b. It increases the surface area available for ATP production, which results in faster cell growth.
The increased surface area of the folds will contain more ATP synthase, allowing for more efficient use of the chemiosmotic gradient and more efficient production of ATP. The observation that plant X grew faster than plant Y supports this conclusion (Unit 2).
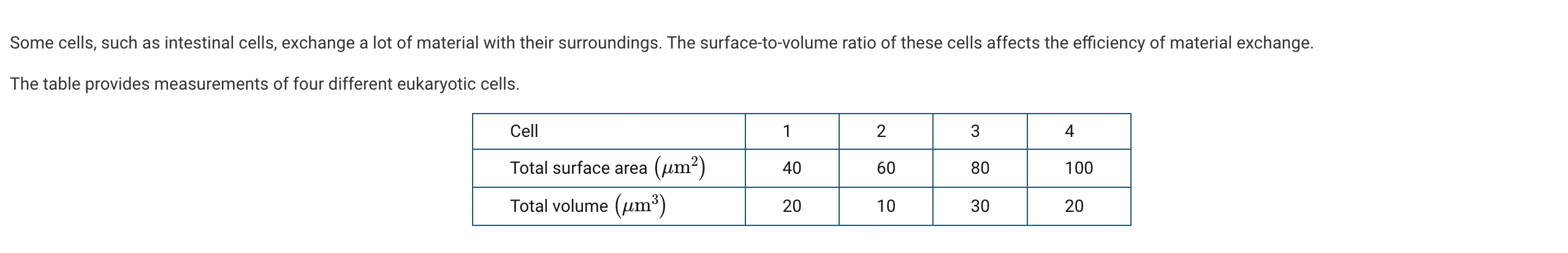
Based on the data, which cell is likely to be most effective in the exchange of materials?
a. cell 1
b. cell 2
c. cell 3
d. cell 4
b. cell 2
The surface area to volume calculation is 60/10=6. Of the four cells, this one has the highest ratio of surface area to volume and is likely to be most effective in the exchange of materials (Unit 2).
Hereditary spherocytosis (HS) is a disorder of red blood cells that causes the cells to be smaller and spherical instead of having the usual flattened, biconcave shape. The average diameter of normal red blood cells is 7.2𝜇m, and the average diameter of red blood cells in a person with HS was found to be 6.7𝜇m. The normal red blood cell has an average surface area of 136𝜇m2 and an average volume of 91𝜇m3.
Which of the following provides an accurate calculation of the surface area to volume ratio of an HS red blood cell, as well as a prediction of its effect on the efficient transferring of oxygen compared to a normal red blood cell?
a. The ratio is 0.45, and the cells are more efficient at transferring oxygen.
b. The ratio is 1.12, and the cells are less efficient at transferring oxygen.
c. The ratio is 0.89, and the cells are less efficient at transferring oxygen.
d. The ratio is 141, and the cells are more efficient at transferring oxygen.
c. The ratio is 0.89, and the cells are less efficient at transferring oxygen
The correct calculation of the surface area to volume ratio of the HS cell is 0.89. This ratio is less than the ratio found in a normal red blood cell, 1.49, so the cell would be less efficient at transferring oxygen (Unit 2).
Stomata are pores on the surfaces of the leaves and stems of plants that regulate gas exchange between the plants and the atmosphere.
Researchers found that the stomata density on the leaves of a species of plant change as the concentration of CO2 in the atmosphere changes. When grown at 350 ppm CO2 the plant has an average density of 300 stomata per mm2, but when grown at 400 ppm CO2 the plant has an average density of 250 stomata per mm2.
Which of the following best describes how the ratio of the density of stomata (stomata per mm2) per CO2 concentration (ppm CO2) changes as the CO2 concentration increases?
a. The ratio decreases from 0.86 to 0.63, because fewer stomata are needed at higher CO2 concentrations.
b. The ratio decreases from 1.6 to 1.2, because fewer stomata are needed at higher CO2 concentrations.
c. The ratio increases from 0.63 to 0.86, because more stomata are needed at higher CO2 concentrations.
d. The ratio increases from 1.2 to 1.6, because more stomata are needed at higher CO2 concentrations.
a. The ratio decreases from 0.86 to 0.63, because fewer stomata are needed at higher CO2 concentrations.
The ratio of 300 stomata per mm2 to 350 ppm CO2 is 0.86, and the ratio of 250 stomata per mm2 to 400 ppm CO2 is 0.63. This reflects that fewer stomata are needed as the concentration of CO2 increases (Unit 2).
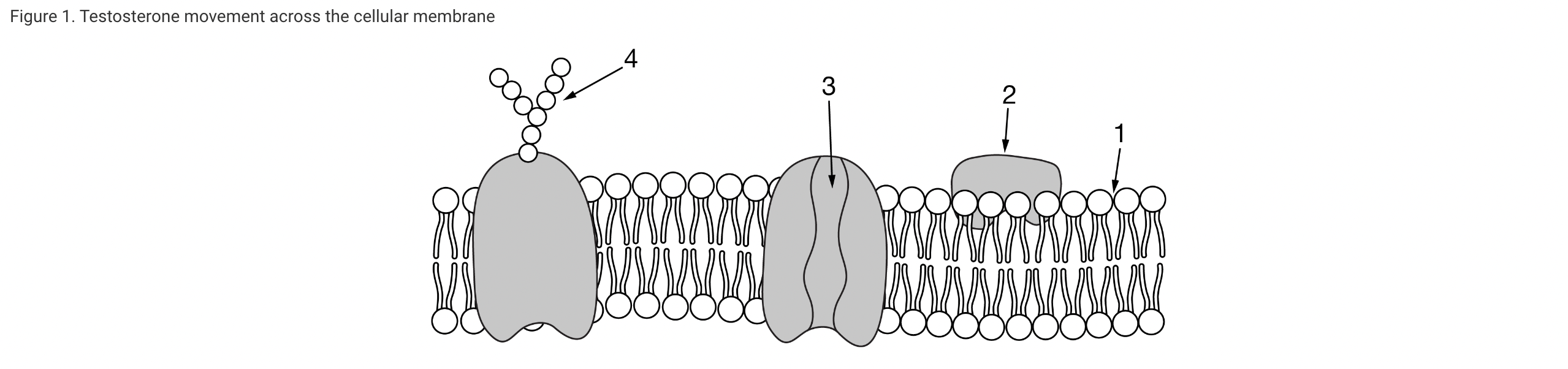
Testosterone is a small steroid hormone that is important in cell signaling. Which of the following describes where testosterone enters a cell and why it is able to cross at that point?
a. 1, testosterone is nonpolar and can diffuse through the membrane.
b. 2, testosterone covalently binds to a surface protein and transports into the cell.
c. 3, testosterone dissolves in water and flows through the channel.
d. 4, testosterone is filtered out of the extracellular fluid and taken into the cell by endocytosis.
a. 1, testosterone is nonpolar and can diffuse through the membrane.
Steroids such as testosterone are hydrophobic lipids. Therefore, testosterone can cross the hydrophobic inner region of the phospholipid bilayer (Unit 2).
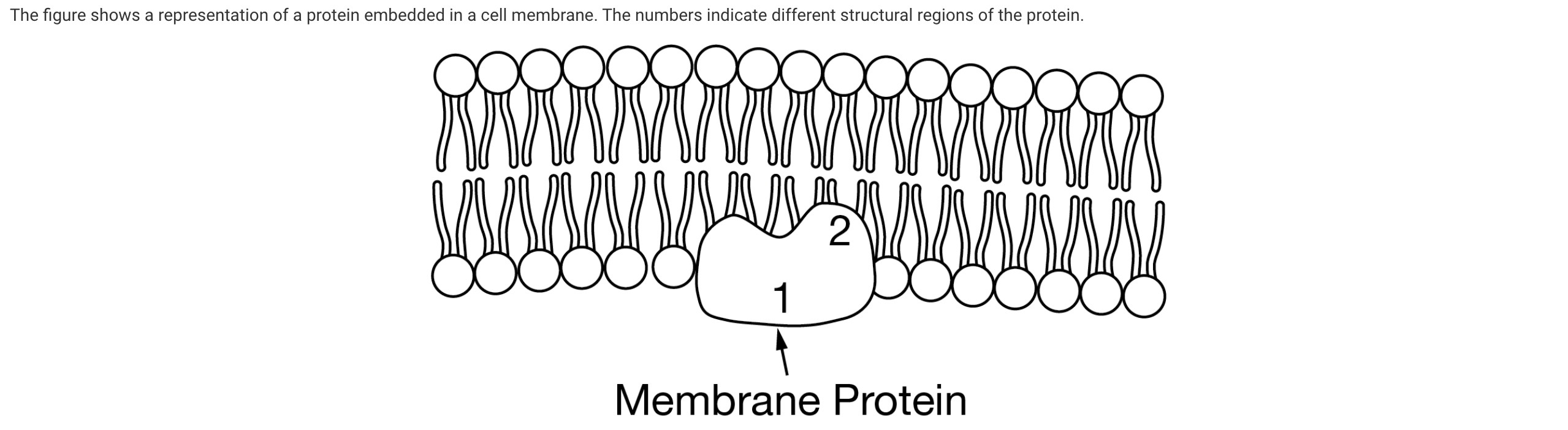
Based on the figure, which of the following statements best describes the relationship between regions 1 and 2 of the protein?
a. Region 1 is hydrophilic because it interacts with the interior of the membrane, whereas region 2 is hydrophobic because it interacts with an aqueous environment.
b. Region 1 is hydrophilic because it interacts with an aqueous environment, whereas region 2 is hydrophobic because it interacts with the interior of the membrane.
c. Region 1 is hydrophobic because it interacts with the interior of the membrane, whereas region 2 is hydrophilic because it interacts with an aqueous environment.
d. Region 1 is hydrophobic because it interacts with an aqueous environment, whereas region 2 is hydrophilic because it interacts with the interior of the membrane.
b. Region 1 is hydrophilic because it interacts with an aqueous environment, whereas region 2 is hydrophobic because it interacts with the interior of the membrane.
A cell membrane is a phospholipid bilayer that separates one aqueous environment from another. The interior of a phospholipid bilayer is a hydrophobic environment. Because region 1 interacts with the aqueous environment on one side of the phospholipid bilayer, it is most likely hydrophilic. Because region 2 interacts with the interior of the phospholipid bilayer, it is most likely hydrophobic (Unit 2).
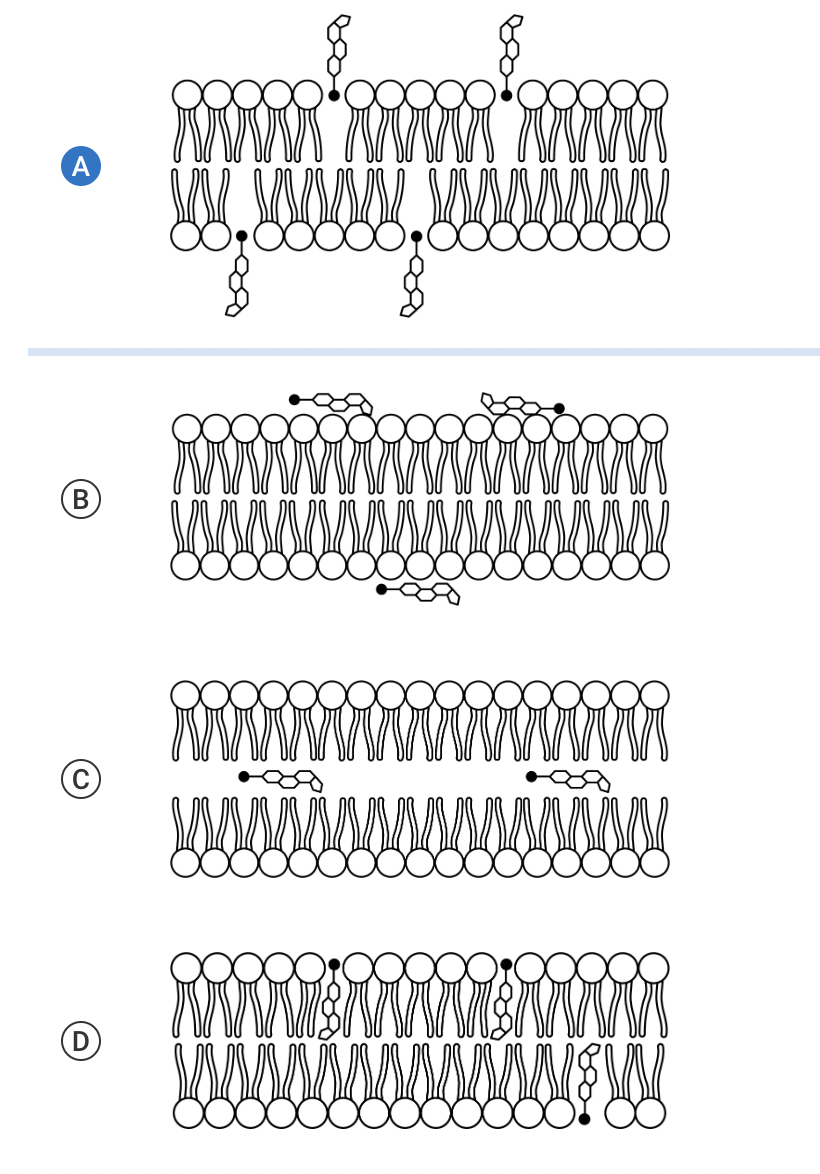
Cholesterol is a naturally occurring substance that helps regulate the fluidity of a cell’s plasma membrane. A cholesterol molecule can be represented as having a polar head and a nonpolar region. Which of the following models shows how cholesterol molecules most likely interact with the phospholipid bilayer of a cell’s plasma membrane?
a, b, c, d
d.
The model correctly shows the polar heads of the cholesterol molecules interacting with the polar heads of the phospholipids. Also, the model correctly shows the nonpolar regions of the cholesterol molecules interacting with the hydrophobic interior of the phospholipid bilayer (Unit 2).
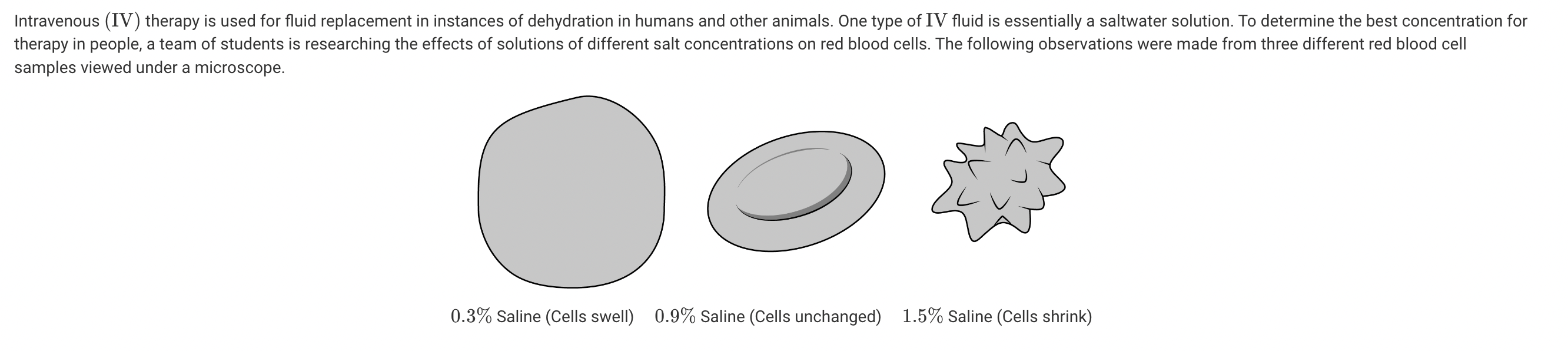
The team wants to extend the research project. What should the team of students do next to obtain data that are more conclusive?
a. Repeat the process with other salt concentrations.
b. Develop a model to explain why the cells react differently to different salt concentrations.
c. Repeat the process using red blood cells from other animals.
d. Develop an experimental procedure that uses a stain that makes the organelles of red blood cells more visible.
a. Repeat the process with other salt concentrations.
The figures illustrate the extremes of hypotonic (swollen cells) and hypertonic (shriveled cells) environments, so an extension would be to investigate the small range of acceptable salt concentrations in IV fluid solutions (Unit 2).
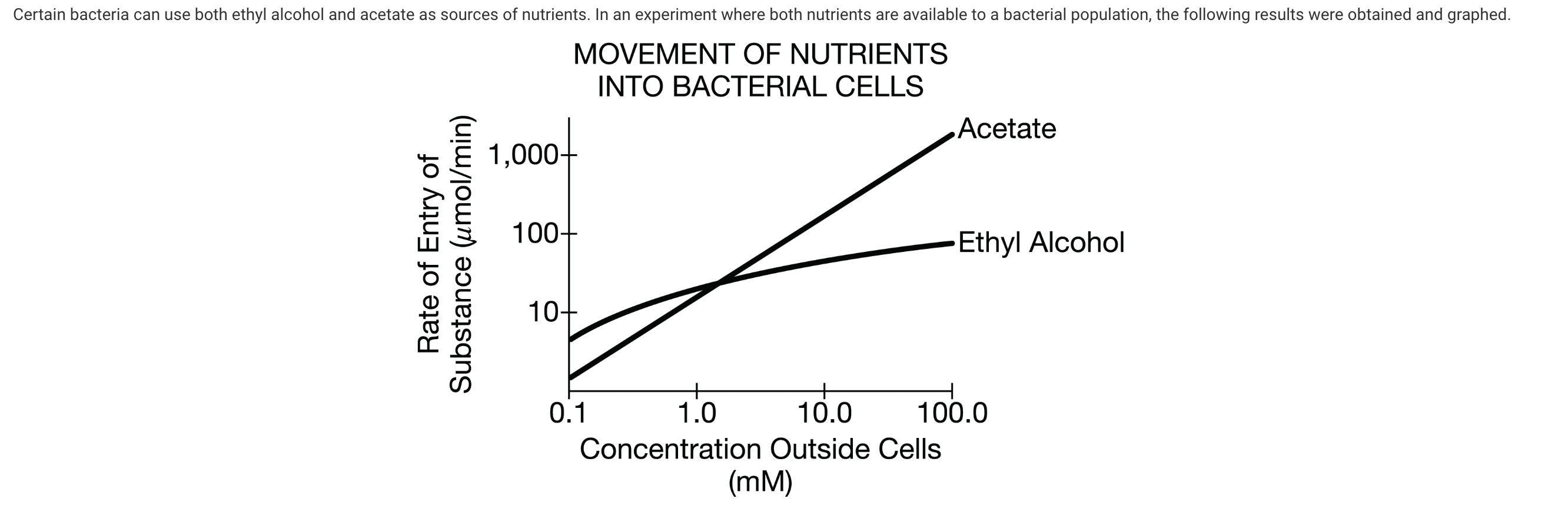
What additional procedure would best help determine whether these movements are due to active transport or to passive transport?
a. Repeat the original experiment, but at three different temperatures. Compare the transport rates among the three temperatures.
b. Repeat the original experiment, but add a substance known to block movement of molecules across aquaporins. Compare the rates on the two graphs.
c. Use two additional treatments, one containing only ethyl alcohol and one containing only acetate. Compare the graphs of these two treatments with the original graph.
d. Use two additional treatments, one containing only ethyl alcohol and one containing only acetate. Include a substance known to block ATP use by the plasma membrane. Compare the graphs of these two treatments to the original graph.
d. Use two additional treatments, one containing only ethyl alcohol and one containing only acetate. Include a substance known to block ATP use by the plasma membrane. Compare the graphs of these two treatments to the original graph.
Active transport requires the availability of ATP. If the nutrients are actively transported, blocking ATP use should reduce the rate of transport along the whole concentration range. If these two graphs are the same as the original graph, the transport is passive. Separation of the nutrients eliminates the possibility that the two transport processes could interfere with each other (Unit 2).
Researchers investigate the transport of a certain protein into cells by endocytosis. In an experiment, the researchers incubate the cells in the presence of the protein and measure the amount of the protein that is absorbed into the cells over a five-minute period.
Based on their observations, what should the researchers do to further clarify how the availability of the protein outside the cells affects the rate of endocytosis of the protein?
a. Incubate the cells in the absence of the protein.
b. Incubate the cells in the presence of several different proteins.
c. Incubate the cells in the presence of several different concentrations of the protein.
d. Incubate the cells in the presence of the protein for several different lengths of time.
c. Incubate the cells in the presence of several different concentrations of the protein.
Changing the concentration of the protein will change the availability of the protein outside the cells (Unit 2).
Lysosomes digest food particles brought into a cell by endocytosis. After a vesicle containing food particles fuses with a lysosome, H+ ions are transported into the lysosome from the cytosol. This significantly lowers the pH of the lysosome relative to the cytosol and activates the enzymes that digest the particles.
Which of the following best predicts what will happen to the lysosomal enzymes if the proteins that transport H+ ions from the cytosol into the lysosome are damaged?
a. The lysosomal enzymes will not become active, since there will be no active transport of H+ ions.
b. The lysosomal enzymes will not become active, since H+ ions will diffuse out of the lysosome.
c. The lysosomal enzymes will become active, since facilitated diffusion will move H+ ions into the lysosome.
d. The lysosomal enzymes will become active, since passive diffusion will move H+ ions into the lysosome.
a. The lysosomal enzymes will not become active, since there will be no active transport of H+ ions.
Since the pH of the lysosome has to become lower than that of the cytosol, active transport is needed to move H+ ions into the lysosome, against the H+ ion concentration gradient, to activate the enzymes (Unit 2).
Which of the following transport mechanisms will be affected most directly by a temporary shortage of ATP molecules inside the cell?
a. The movement of water molecules through aquaporins
b. The diffusion of oxygen molecules across the plasma membrane
c. The transport of glucose molecules against a concentration gradient
d. The facilitated diffusion of Ca2+ ions into the cell
c. The transport of glucose molecules against a concentration gradient
The active transport of glucose molecules against a concentration gradient requires an input of energy. Biological processes that require an input of energy typically incorporate the hydrolysis of ATP, an energy-rich molecule (Unit 2).
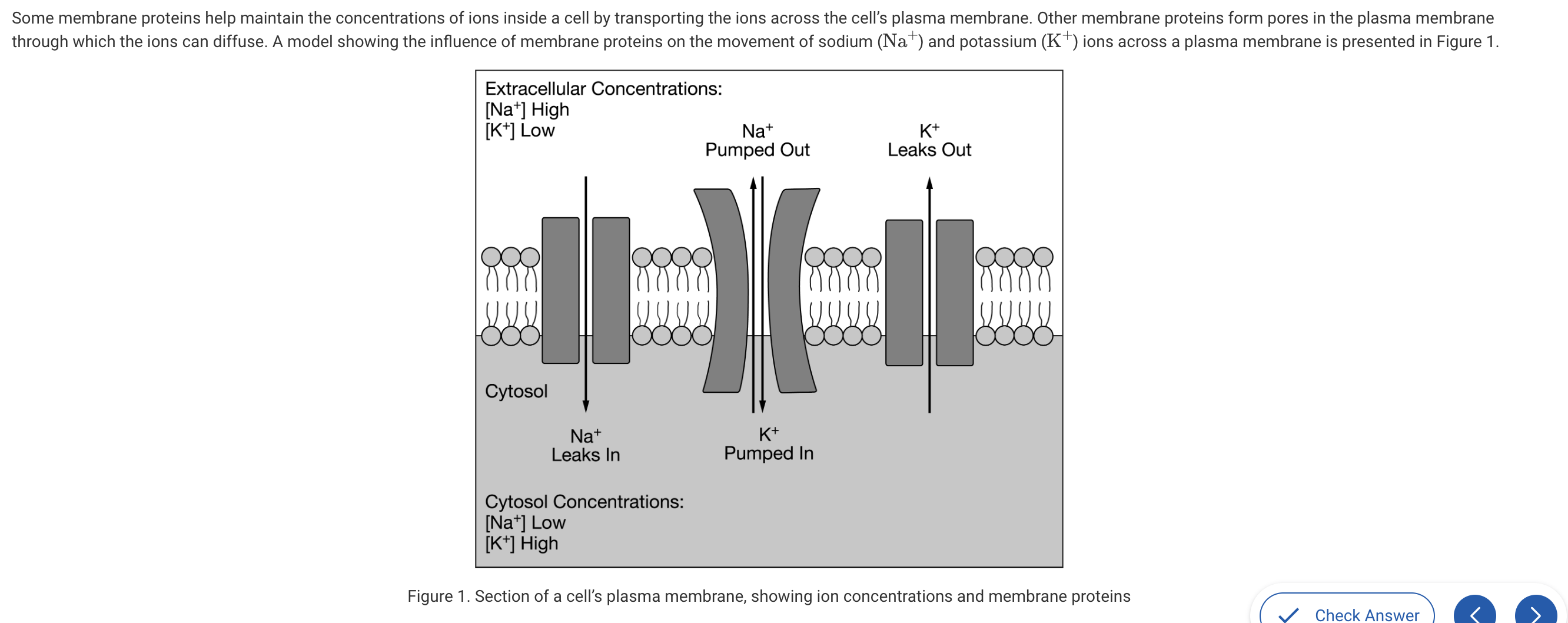
Based on the model presented in Figure 1, which of the following changes will most likely result from a depletion of available ATP stores inside the cell?
a. The Na+ concentration outside the cell will increase.
b. The Na+concentration inside the cell will increase.
c. The K+ concentration inside the cell will increase.
d. The K+ concentration outside the cell will decrease.
b. The Na+concentration inside the cell will increase.
Without ATP, the Na+ ions will continue to leak into the cell but will not be pumped out of the cell, resulting in a concentration increase inside the cell (Unit 2).
A study was conducted to understand the factors controlling the rate at which molecules or ions travel across cell membranes. An artificial membrane was created that was composed of a phospholipid bilayer only. The speed at which various substances crossed this membrane was measured.
Some substances can pass through an actual cell membrane much faster than they passed through the artificial membrane in this study. Which of the following statements best explains this finding?
a. Actual cell membranes have a much thicker phospholipid bilayer than the artificial membrane does.
b. Actual cell membranes have a variety of proteins embedded in the membrane that are absent in the artificial membrane.
c. Hydrophobic substances spend more time between the two layers of phospholipid in the artificial membrane than they do between the layers in an actual membrane.
d. Hydrophilic substances spend more time attached to the polar region of the phospholipids in the artificial membrane than they do attached to the polar region of the phospholipids in an actual membrane.
b. Actual cell membranes have a variety of proteins embedded in the membrane that are absent in the artificial membrane.
Actual cell membranes have a variety of embedded proteins that increase the movement of substances across the membrane by both active and passive transport (Unit 2).
A magnesium sulfate solution taken orally can cause a net movement of water into the large intestine, which results from water molecules diffusing through aquaporins embedded in the cells of the intestinal lining.
By which of the following mechanisms do the water molecules most likely move into the large intestine?
a. By passive transport from an area of low osmolarity to an area of high osmolarity
b. By passive transport from an area of high osmolarity to an area of low osmolarity
c. By active transport from an area of low osmolarity to an area of high osmolarity
d. By active transport from an area of high osmolarity to an area of low osmolarity
a. By passive transport from an area of low osmolarity to an area of high osmolarity
Based on the information presented, the water molecules move through aquaporins by diffusing from an area of low osmolarity to an area of high osmolarity, which is an example of passive transport (Unit 2).
Aldosterone (a steroid hormone) is a small, nonpolar, hydrophobic molecule that enters a target cell by moving across the plasma membrane, down a concentration gradient.
Based on the information presented, how does aldosterone most likely enter target cells?
a. By simple diffusion because aldersterone can enter a cell by moving across the plasma membrane and moving down the concentration gradient.
b. By facilitated diffusion because aldersterone would need the assistance of a protein channel to move across the plasma membrane.
c. By active transport because aldersterone would need energy to move across the plasma membrane.
d. By endocytosis because aldersterone would need to be transported by vesicle across the plasma membrane
a. By simple diffusion because aldersterone can enter a cell by moving across the plasma membrane and moving down the concentration gradient.
Small, nonpolar, hydrophobic molecules can enter a cell by moving across the plasma membrane and down a concentration gradient by simple diffusion (Unit 2).
Some viral infections can lead to the rupture of the lysosome membrane. Which prediction of the effect of this disruption of cellular compartmentalization is most likely correct?
a. Enzymes will be released that will specifically target the virus.
b. Cellular osmotic concentrations will change, preventing viral entry into the cell.
c. Hydrolytic enzymes will be released, which will cause cell death.
d. Intracellular digestion of organic materials will increase, which will increase the energy available to the cell for fighting the virus.
c. Hydrolytic enzymes will be released, which will cause cell death.
Hydrolytic enzymes will be released, resulting in cell death and preventing further viral reproduction (Unit 2).
Gaucher disease is an inherited disorder in which cells of the body are unable to break down a particular type of lipid, resulting in a buildup of the lipid in some tissues and organs.
Based on the information provided, Gaucher disease results most directly from a defect in the function of which of the following organelles?
a. The smooth endoplasmic reticulum
b. The nucleus
c. The lysosome
d. The mitochondrion
c. The lysosome
The lysosome contains specific enzymes used to break down a variety of molecules and cellular waste products. A defect in the function of the lysosomal enzymes that are needed to break down lipids is the most direct cause of Gaucher disease (Unit 2).
Researchers have proposed a model of chloroplast evolution. According to the model, chloroplasts evolved from a small prokaryotic organism that was engulfed by an ancestral eukaryote. The engulfed prokaryote then formed an endosymbiotic relationship with the eukaryotic host.
Which of the following observations best supports the model?
a. Chloroplasts are separated from other subcellular compartments by semipermeable membranes.
b. Prokaryotic and eukaryotic organisms both acquire nutrients from the surrounding environment.
c. Eukaryotes evolved after prokaryotes and have more complex structures.
d. Chloroplasts and some prokaryotes share similar photosynthetic reactions.
d. Chloroplasts and some prokaryotes share similar photosynthetic reactions.
Photosynthesis is a feature of only a limited number of organisms, including cyanobacteria, algae, and plants. The fact that this specialized process is similar in prokaryotes, such as cyanobacteria, and in eukaryotes, such as algae and plants, provides evidence in support of the model (Unit 2).
Which of the following observations best supports the claim that mitochondria evolved from once-free-living prokaryotic cells by the process of endocytosis?
a. Mitochondria produce ATP.
b. Mitochondria contain proteins.
c. Mitochondria exchange substances with the cytosol.
d. Mitochondria are surrounded by a double membrane.
d. Mitochondria are surrounded by a double membrane.
The double membranes of mitochondria provide evidence that an ancestor of mitochondria, which was most likely a type of free-living aerobic bacterium, was ingested via endocystosis by a primitive eukaryotic cell (Unit 2).
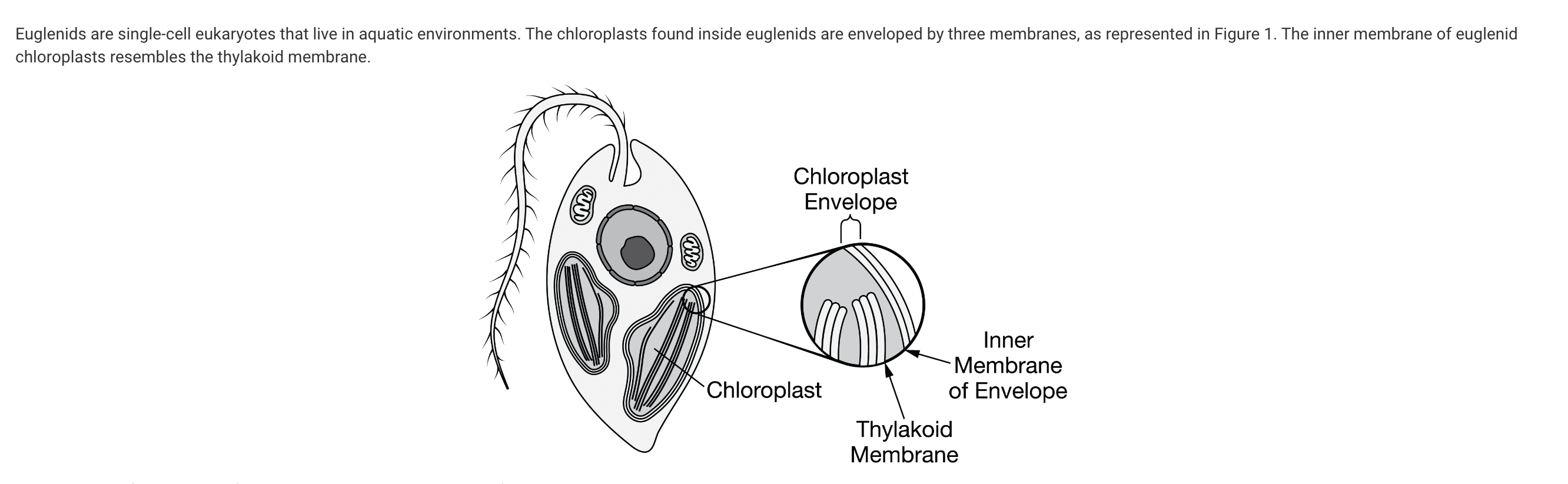
Which of the following claims about the origin of the euglenid chloroplast is best supported by the three-membrane structure of the envelope?
a. It originated from the spontaneous assembly of organic molecules into a lipid bilayer inside a free-living prokaryote.
b. It originated from the fusion of the plasma membranes of two different free-living photosynthetic prokaryotes.
c. It originated from the incorporation of a photosynthetic prokaryote into a eukaryotic cell by a single endosymbiotic event.
d. It originated from the incorporation of a photosynthetic prokaryote into a eukaryotic cell by two endosymbiotic events.
d. It originated from the incorporation of a photosynthetic prokaryote into a eukaryotic cell by two endosymbiotic events.
The three-membrane structure of the chloroplast envelope best supports the claim presented in this answer choice. The three-membrane structure most likely resulted from two endosymbiotic events, with the middle membrane originating from the first event and the outer membrane originating from the second event (Unit 2).
Beetroot cells contain a family of dark red pigments called betalains. The selectively permeable nature of the beetroot cells keeps the internal environment of the cell separate from the external environment of the cell. Researchers are interested in determining whether the selective permeability of beetroot cells is due to the cell membrane or if it is due to the cell wall.
Exposure to cellulase is known to damage the structure of the cell wall. An experiment is set up in which beetroot cells are placed in an aqueous solution with cellulase and in one without cellulase.
Which of the following results best refutes the alternative hypothesis that selective permeability is a consequence of the cell wall?
a. When beetroot cells are placed in a solution with cellulase, the solution turns dark red.
b. When beetroot cells are placed in a solution with cellulase, the solution remains clear.
c. When beetroot cells are placed in a solution, it turns dark red with or without cellulase present.
d. Since plant cells contain cell membranes, not cell walls, the alternate hypothesis cannot be tested.
b. When beetroot cells are placed in a solution with cellulase, the solution remains clear.
Cellulase digests cellulose and damages the structure of the cell wall, not the cell membrane. The lack of color change in the solution indicates that the betalain is not leaking out of the beetroot cells even though the cell wall has been damaged. This refutes the alternative hypothesis (Unit 2).
b. When beetroot cells are placed in a solution with cellulase, the solution remains clear.
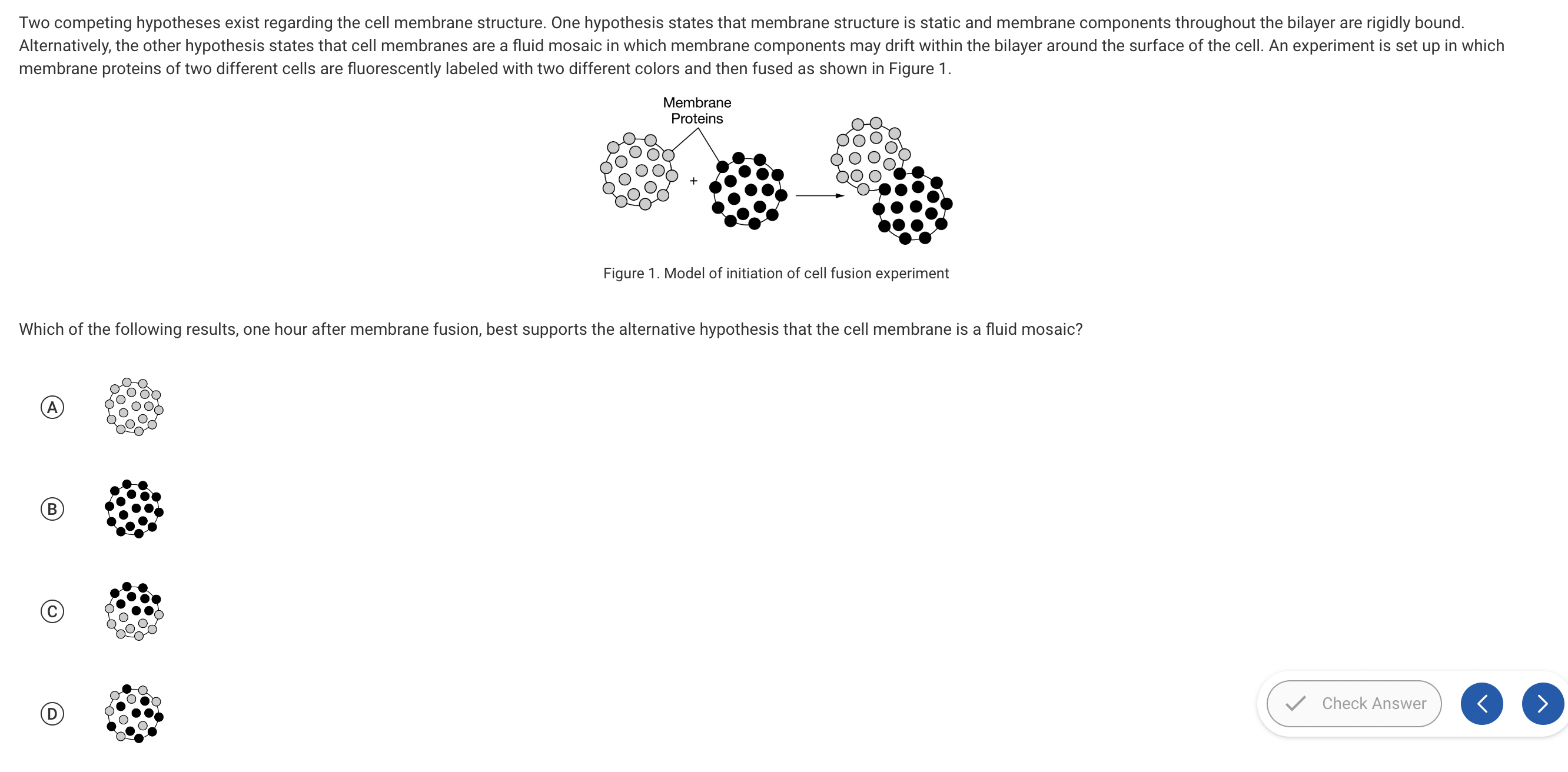
a, b, c, d
d.
The membrane proteins from each cell have mixed and drifted within the bilayer, which supports the fluid mosaic model of cell membranes. Cell membranes consist of a structural framework of phospholipid molecules that is embedded with proteins and steroids that may flow around the surface of the cell within the bilayer (Unit 2).
The cell membrane is selectively permeable due to its structure. Thus, the internal environment of the cell is distinct from the external environment of the cell. One biologist hypothesizes that small nonpolar molecules readily pass through the membrane. Another biologist alternatively hypothesizes that these types of molecules require channel and transport proteins that are embedded in the membrane in order to move across the membrane.
Which of the following data would best refute this alternative hypothesis?
a. Ethanol is found in the cytosol of cells when they are briefly exposed to a ten percent ethanol solution.
b. Cells become oxygen deficient when membrane protein activity is blocked.
c. CO2 and N2 movement in and out of cells is unaffected when membrane protein activity is blocked.
d. Sodium ions cannot move across the cell membrane when membrane protein activity is blocked.
c. CO2 and N2 movement in and out of cells is unaffected when membrane protein activity is blocked.
Both CO2 and N2 are small nonpolar molecules. Their ability to enter and leave cells normally when membrane protein activity is blocked would refute the alternative hypothesis (Unit 2).