Chemistry H Midterm Practice Problems (Use Periodic Table!)
1/22
Earn XP
Description and Tags
Go in order!
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
23 Terms
Ionic Compound Practice!
Name, write charges, and/or write formulas for each!
🙂
Mg3P2
Name: Magnesium Phosphide
Cation:Mg(2+)
Anion: P(3-)
Potassium Oxide
Cation: K(+)
Anion: O(2-)
Formula: K2O
Copper (III) sulfide
Cation (found through calculation): Cu(3+/III)
Anion: S(2-)
Formula: Cu2S3
Bond Fe and SO4
Name: Iron(II) Sulfate
Cation (found through calculation): Fe(2+/II)
Anion: SO4(2-)
Formula: FeSO4
PbSO4
Name: Lead(II) Sulfate
Cation (found through calculation): Pb(2+/II)
Anion: SO4(2-)
Bohr Diagrams!
😲
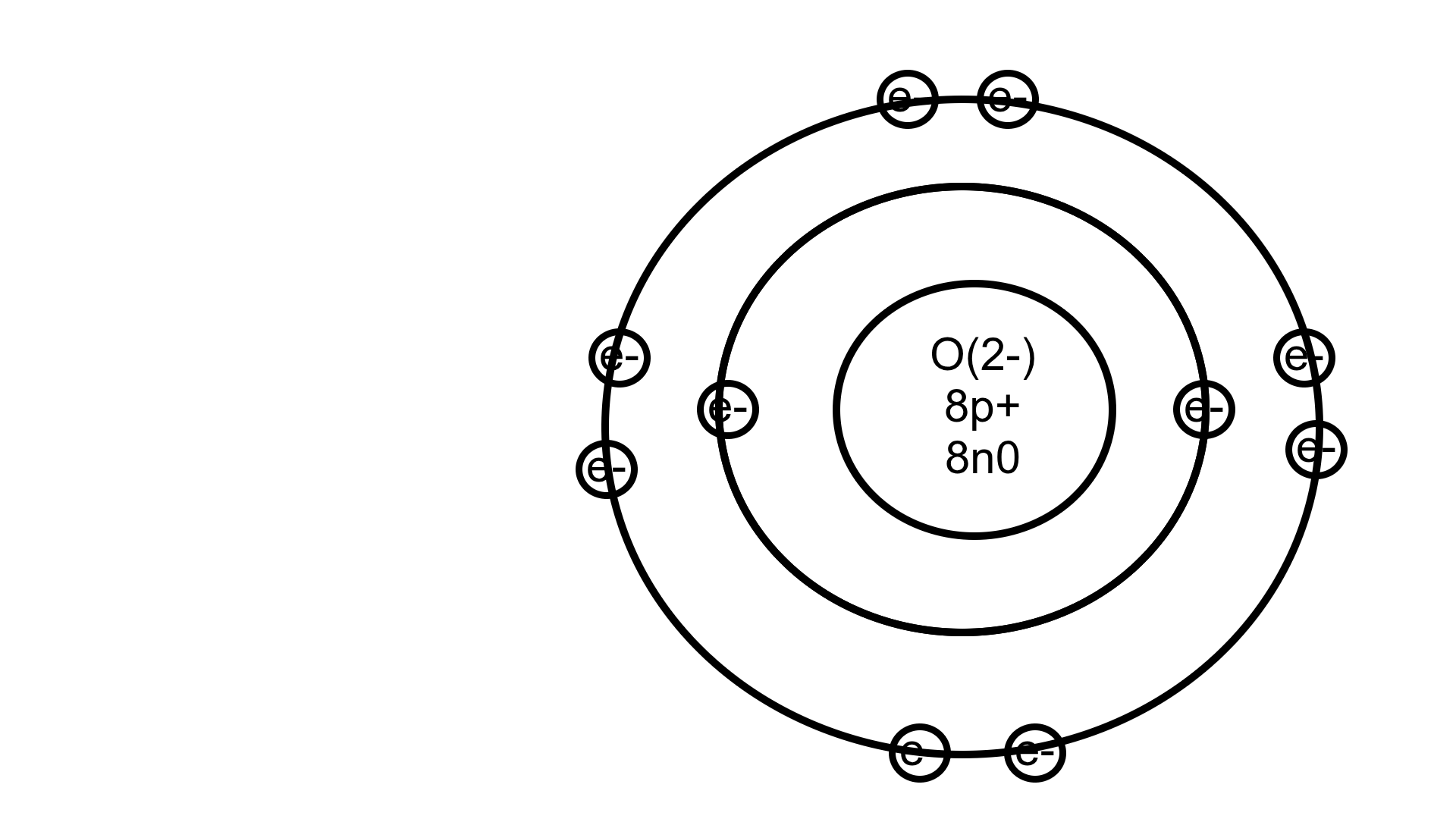
Draw a Bohr diagram for an ion of O with a -2 charge and 8n
(8p+, 8n0, 10e-)
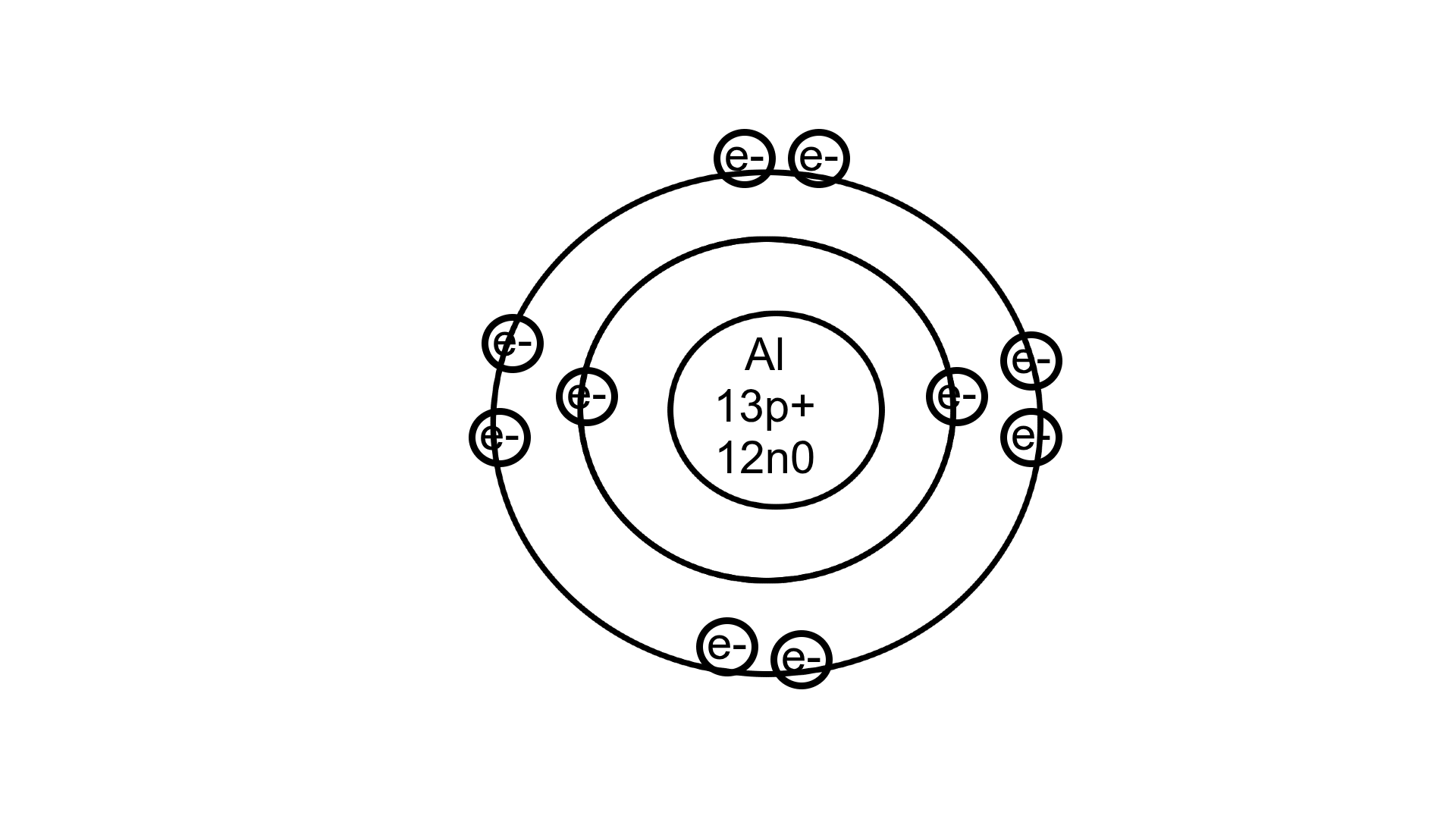
Draw a Bohr Diagram for Aluminum-27
(13p+, 14n0, 13e-)
Physical and Chemical properties and Changes, Elements, Compounds, and Mixtures
😛
Label as a chemical change or a physical change:
Rusting
Food spoiling
Melting ice
Burning paper
boiling water
Dissolving sugar in water
Rusting C
Food spoiling C
Melting ice P
Burning paper C
Boiling water P
Dissolving sugar in water P
Classify as element, compound, or mixture:

a) mixture (between elements)
b) element
c) compound
Classify as element, compound, or mixture:
Na + Cl2
Fe + KBr
AlPO4
P4
Na + Cl2 Mixture (between elements)
Fe + KBr Mixture (between elements and compounds)
AlPO4 Compound
P4 Element
Electronegativity practice
😉
Si and P
Difference: 0.3
Bond type: nonpolar covalent
Br and K
Difference: 2.2
Bond type: ionic
I and Rb
Difference: 0.9
Bond type: polar covalent
Covalent Compounds
☹
N2O3
Dinitrogen Trioxide
SCl5
Phosphorus Pentachloride
Tetraphosphorus Decoxide
P4O10
Nitrogen Monoxide
NO
I will put in more as we get practice Problems!
🤔