Nuclear Physics
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
62 Terms
What model did scientists have before the nuclear model of the atom?
The Plum Pudding model
Describe the Plum Pudding model.
Proposed that the atom was an uniform mass of positive charge dotted with negative charge with the overall charge being zero- neutral.
Describe the Alpha particle scattering experiment by Rutherford.
Fast moving, positively charged Alpha radiation emitted by radioactive material.
Fired at gold thin sheet.
The detector made of Zinc Sulfide, surrounding, detected the deflected alpha particles and produced a light flash whenever the particles hit the detector.
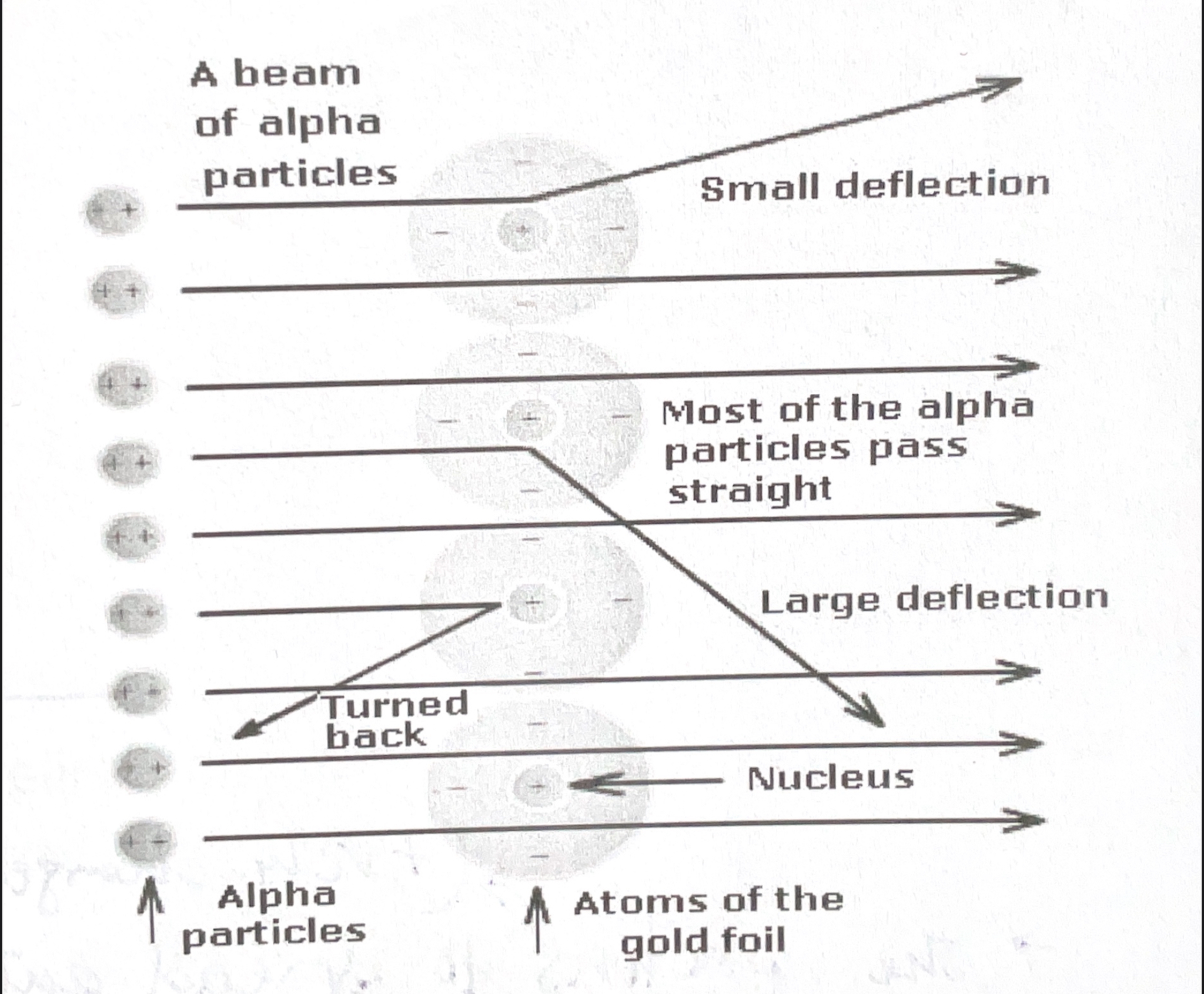
What observations did the experiment have?
The most of the particles traveled passed through the sheet without any deflection.
A some particles deflected off at angles less than 90 degrees.
Only few particles (1/8000) deflected at angles greater than 90 degrees.
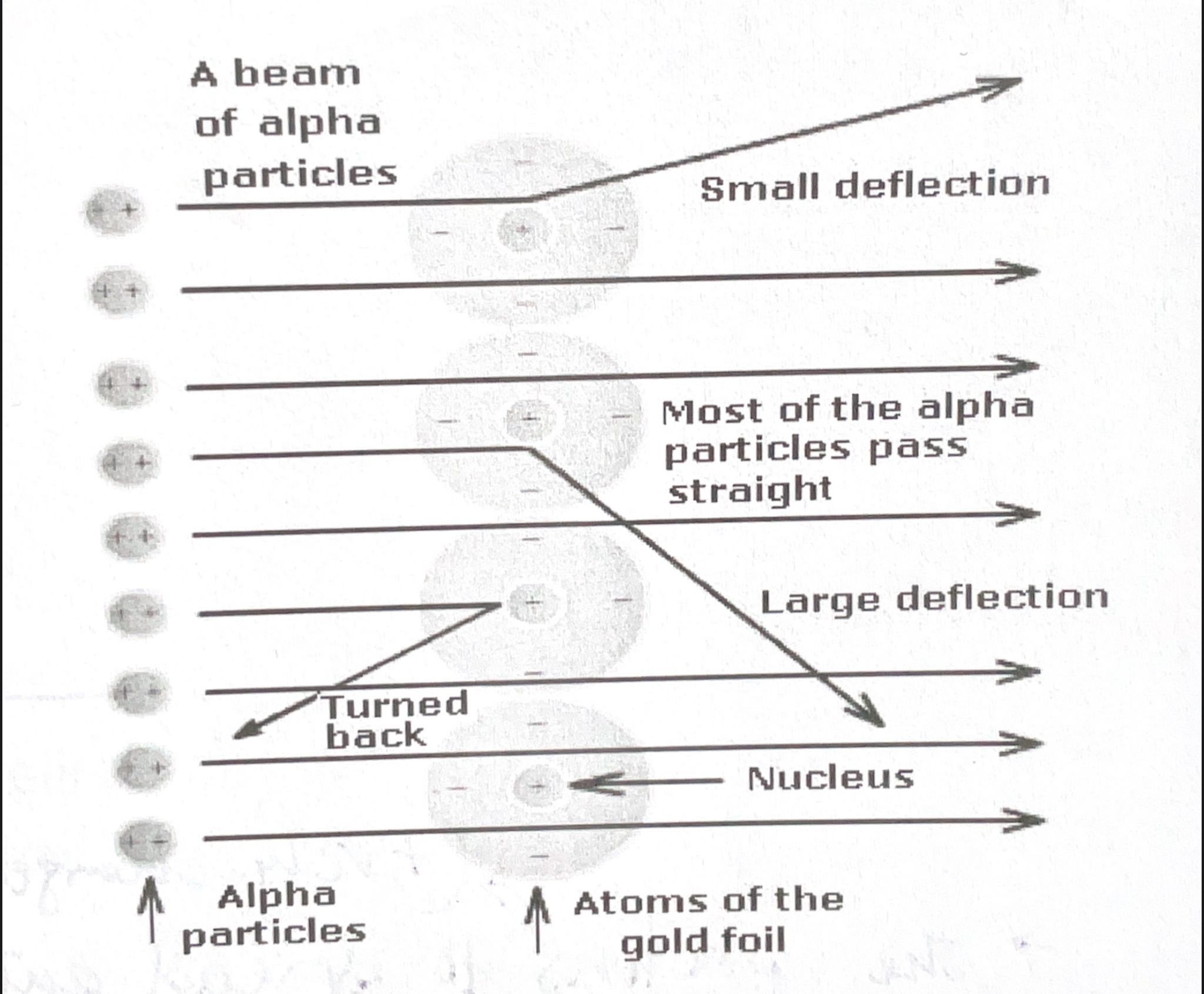
What deductions did the experiment have?
Most of the atom’s mass is concentrated in centre of the atom, in the nucleus (with smaller volume and higher density).
The nucleus is positively charged.
The nucleus’ diameter (10^-15) is considerably smaller than the diameter of the atom (10^-10), hence the atom is mostly made of empty space.
The main differences between the Plum Pudding model and the Nuclear model?
Plum Pudding model :
The positively charged spheres (protons) are spread out in the atom to form a positive cloud, which has negatively charged electrons scattered on those spheres.
The nuclear model:
The positively charged spheres is in the centre of the atom and is small.
The atom is mostly made of empty space.
What result might you have expected if the Plum pudding model was correct?
All alpha particles to be undeflected and pass through the gold sheet.
In Rutherford’s experiment, explain the theory was used when the alpha particles were fired at the gold thin sheet?
The idea of conservation of energy to determine the size of the nucleus’ diameter from the initial kinetic energy of the alpha particle.
The alpha particle must do work against the electrostatic repulsion between itself and the positively charged gold atoms.
As the alpha particles moved, their kinetic energy was converted into electrostatic potential energy when they approached the positively charged gold nuclei.
Large-angle deflections required significant electrostatic repulsion, implying a small, dense nucleus rather than a diffuse “plum pudding” model.
Initial Kinetic Energy of Alpha Particle = Maximum Electrostatic Potential Energy at Closest Approach.
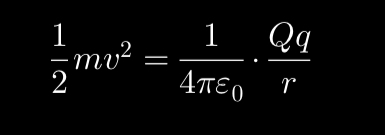
Three characteristics of the Nuclear model of the atom.
Electrons can only occupy certain orbits or energy levels around the nucleus.
Protons are positively charged particles in the centre of the atom, in the nucleus.
Neutrons are neutrally charged particles with a mass very close to protons.
1u = 1.661×10^-27 kg
Define isotopes?
Atoms with same number of protons with different number of neutrons.
Characteristics of strong nuclear force.
Always attractive with maximum strength at around 0.5 fm but the strength falls rapidly beyond this range.
Acts on hadrons.
Binds quarks together due to their ‘colour charge’ - Just like EM forces act on particles with electric charge and will bind together two positively charged particles
Characteristics of weak nuclear force.
Responsible for beta decay and other forms of radioactive decay by mediating interactions that change one type of quark into another.
Acts on all fermions - hadrons and leptons.
Can change one type of particle into another.
Define quark.
A fundamental particle that make up hadrons by experiencing strong nuclear force.
Define hadron.
Any particle made of quarks.
Define a baryon.
Any particle made up of three quarks or antiquarks, for example, neutron(dud), proton(udu).
Define a meson.
Any particle made up of an quark-antiquark pair, for example pi+ = up quark and an anti-down quark.
The quark-antiquark pair doesn’t have to be constituents of each other.
What is an anti-particle?
A particle with opposite charge with same mass as its constituent particle or its pair.
For example, electron’s anti-particle is positron with a positive charge.
Which quarks are the most stable?
Up-quark
Down-quark
What is ‘strange’ about the strange quark?
It’s the only quark with the strangeness of +1 and the anti-strange quark has the strangeness value of -1.
What is the charge of neutrino and an anti neutrino?
0e
What is the charge on an up quark and an anti-up quark?
+2/3 e
-2/3e
What is the charge on a down quark and an anti-down quark?
-1/3e
+1/3e
What is the charge on a tau particle and an anti-tau particle?
-1e
+1e
What is the charge on a charm particle and an anti-charm particle?
+2/3e
-2/3e
What is the baryon number of a quark and it’s anti-quark?
Quark = 1/3
Anti-quark = -1/3
What is the equation of Mass -Energy equivalence?

Equation of Mass defect and Binding energy?

Equation of Nuclear radius and density?

Equation of alpha decay?

Equations of Beta decay?

Equations of Beta minus decay at fundamental level?
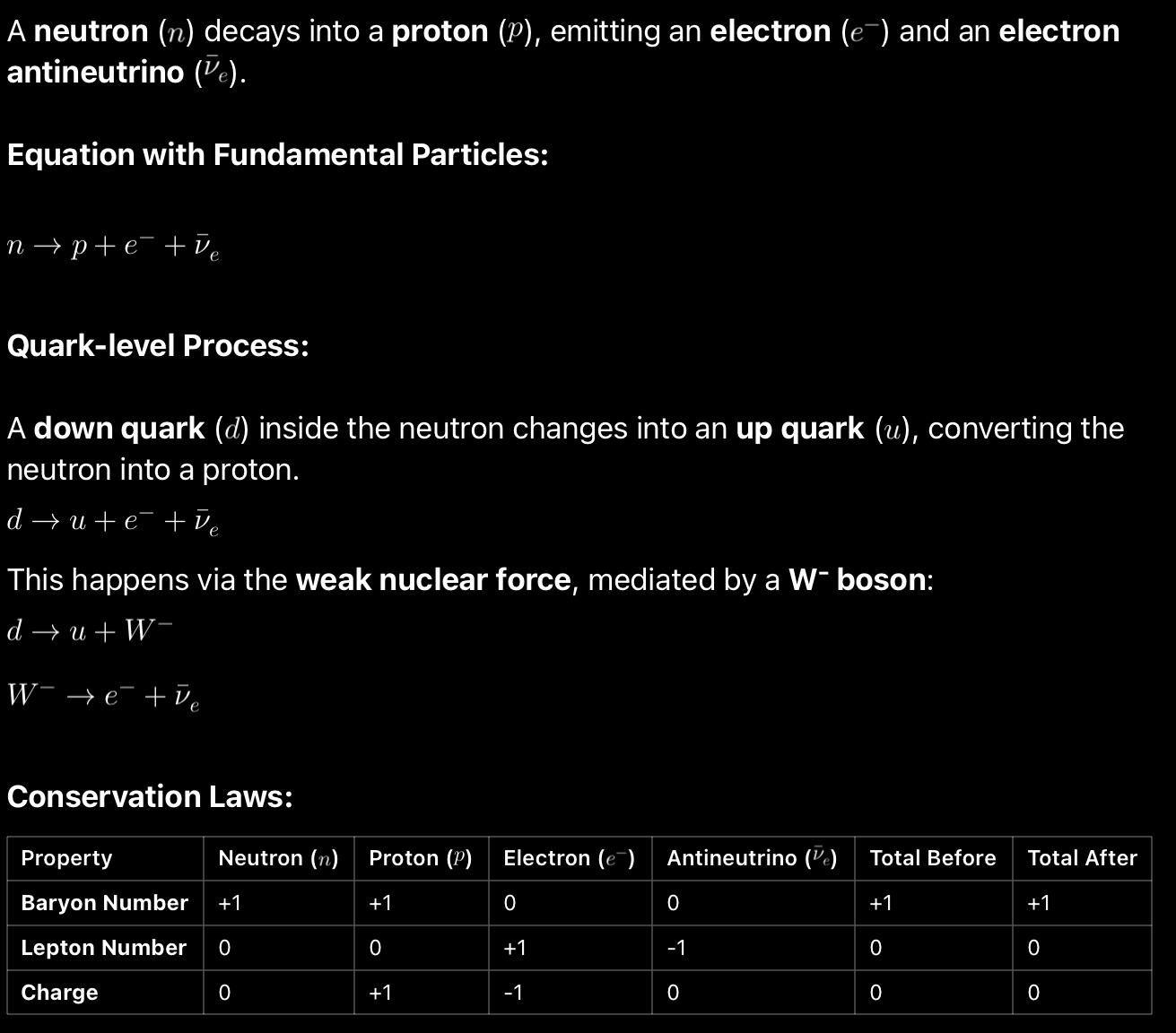
Equations of Beta plus decay at fundamental level?

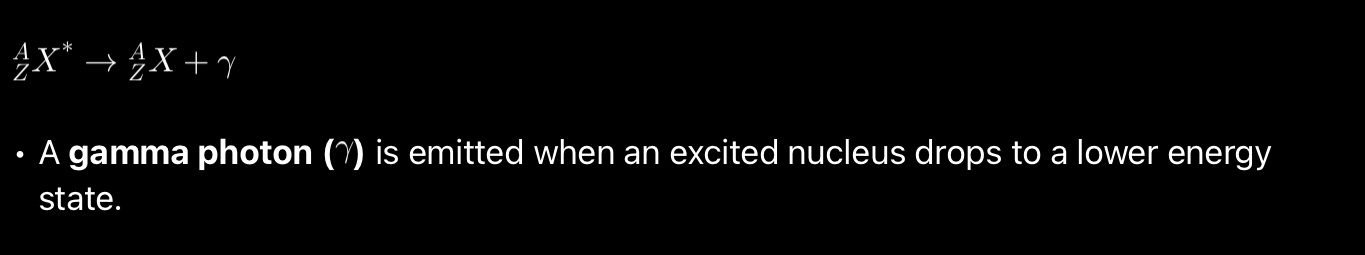
Equation of gamma decay?
Equations of Half life and activity?

Equation of nuclear fission?
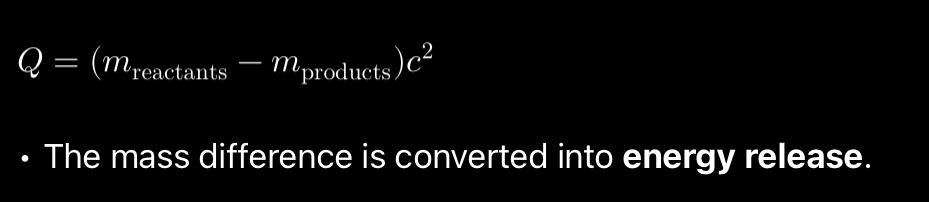
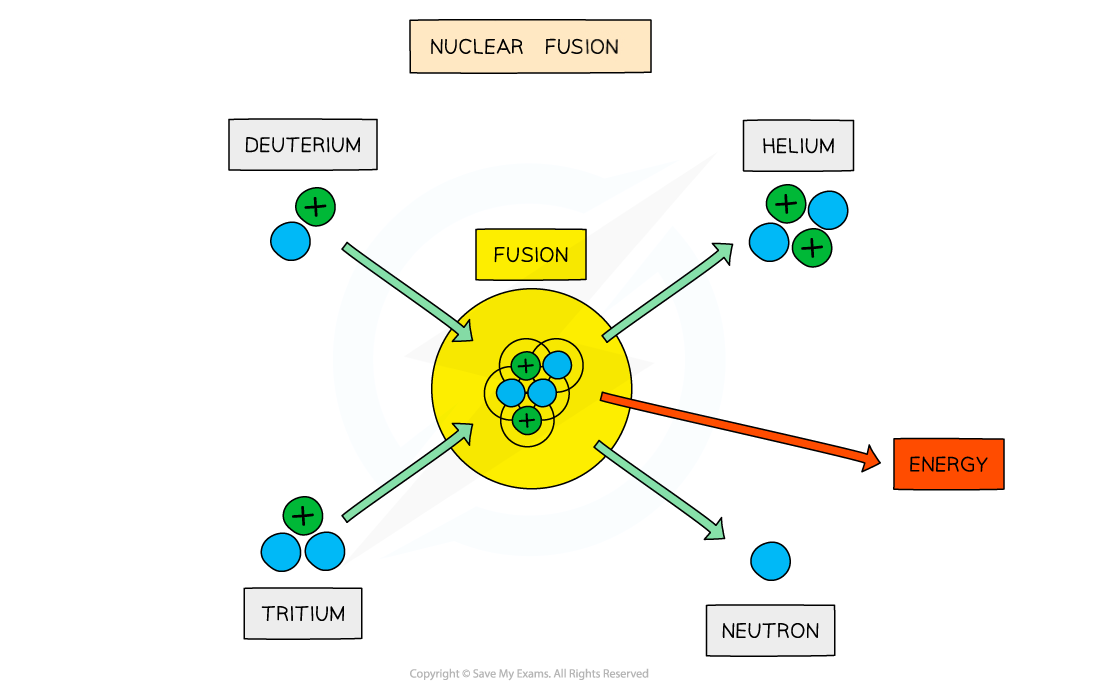
Equation of nuclear fusion?

Beta minus decay.
Beta- minus particle is a high speed electron.
The decay happens when a radioactive nuclei has many neutrons and undergoes beta minus decay for stability.
A neutron turns into a proton, and emits an electron, anti electron neutrino.
A down quark turns into an up quark.
Mass= 1/1840u
Charge= -1e
Speed= 0.99c
Beta plus decay.
A beta plus particle is a high speed positron.
The decay happens when a radioactive nuclei active nuclei has too many protons and undergoes beta plus decay for stability.
A proton is converted into a neutron, and emits a positron, electron neutrino.
An up quark turns into a down quark.
Mass= 1/1840u
Charge= +1e
Speed= 0.99c
Penetration can be blocked using ~ few mm of aluminium sheet.
Alpha decay.
Is considered a particle with a helium nucleus.
Mass= 4u
Charge= +2e
Speed= 0.05c
Alpha particles are larger and most ionising power than both beta and gamma particles.
But have least range of penetration, about ~ few cm (can be blocked by few cm of air or one of paper)
Gamma decay.
High energy photon or EM radiation.
Mass= 0u
Charge= 0e
Speed= c
Gamma decay happens, when a nucleus has too much energy often after an alpha or a beta decay.
Penetration can be blocked using ~ few mm of thick lead block.
Two assumptions of Radio active decay?
Random: which radioactive nuclei and when it will occur cannot be predicted, as each nucleus has an equal chance to decay per unit time.
Spontaneous: the probability of decay of the radioactive nuclei in the sample is unaffected by any external influences. I.e. changes in physical conditions like temperature or pressure.
Activity definition.
Activity (A) is the rate at which a radioactive substance decays. It is defined as the number of decays per second and is measured in becquerels (Bq).
1 Bq = 1 decay per second
Define decay constant?
Decay constant (\lambda = Decay constant (s^{-1})) is the probability that a given nucleus will decay per unit time.
What is relationship between the number of un-decayed nuclei and time.
The number of un-decayed nuclei decreases exponentially over time.
There is an exponential decay as same fraction of nuclei decays each time in equal proportion, regardless of the number of sample.
The larger the lambda (decay constant ), the faster the decay rate as greater number of un-decayed nuclei decays in shorter time period.
So, with larger lambda, the half life is shorter.
What is half-life?
The average time taken by the sample to half. (t1/2)
Lambda = ln(2)/t1/2
Assumption of Mass- energy change of everyday objects.
The change in mass due to energy change is negligible for everyday objects.
How does the change in mass occurs in particles?
The change in mass occurs as a result of beta decay, where the mass- energy is converted into kinetic energy of the particles.
Radioactive decay:
When a nucleus decays, the total mass of the products is less than the original nucleus. The missing mass (mass defect) is converted into energy (kinetic energy of emitted particles or radiation).
Nuclear fission:
The total mass of the fission products and released neutrons is less than the original mass of the nucleus.
The mass difference is converted into energy, which powers nuclear reactors.
Nuclear fusion (combining light nuclei to form one larger nucleus):
The total mass of Helium-4 and the neutron is less than the sum of the deuterium and tritium.
The missing mass is converted into energy, making fusion highly efficient.
Mass change in fundamental particles:
Mass change can also happen at the particle level due to interactions with the weak nuclear force.
Mass- defect definition?
The difference between the mass of a nucleus and sum of the masses of the nucleons is they were to completely be separated.
Binding energy definition?
Binding energy of the nucleus is the minimum energy required to separate the nucleons into its constituent protons and neutrons.
The greater the binding energy, more strongly bonded the nucleons are to each other.

Nuclear fission?
When a larger unstable nuclear splits into two smaller ‘daughter’ nuclei.
The sum of binding energies of the smaller nuclei is greater than of the larger nucleus formed -due to mass lost.
Isotopes of uranium and plutonium both undergo fission and are used as fuels in nuclear power stations.
During fission, when a neutron collides with an unstable nucleus, the nucleus splits into two smaller nuclei (called daughter nuclei) as well as two or three neutrons.
Gamma rays are also emitted.
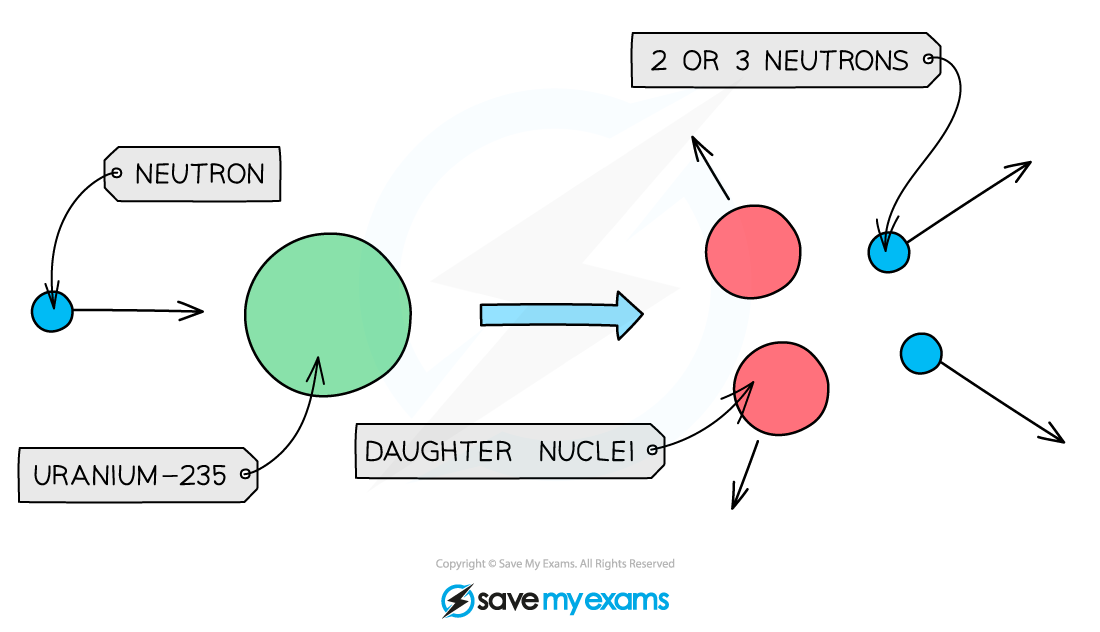
What’s a slow neutron and how is it achieved?
Are those with a mean kinetic energy equal to the thermal energy of particles in the reactor core.
They have the highest chance of causing fission because they are more likely to collide and transfer all their energy to the reactor.
A slow neutron is known as a thermal neutron, which is absorbed by atom undergoing nuclear fission.
A moderator (made up of graphite) is used in the reactor to slow down the incoming fast neutron, so the atom undergoing fission can absorb it.
What does the chain reaction in nuclear fission cause and how can you calculate the number of neutrons produced.
The chain reaction occurs when the initial neutrons cause an exponential increase in the number of neutrons which further causes an exponential growth in the rate of fission reactions.
Simple formula:
N=3^n

What’s the difference between induced and spontaneous fission?
Induced fission:
Usually, for fission to occur the unstable nucleus must first absorb a neutron
Take, for example, uranium-235, which is commonly used as a fuel in nuclear reactors
It has a very long half-life of 700 million years
This means that it would have low activity and energy would be released very slowly
This is unsuitable for producing energy in a nuclear power station
During induced fission, a neutron is absorbed by the uranium-235 nucleus to make uranium-236
This is very unstable and splits by nuclear fission almost immediately
Spontaneous fission:
It is rare for nuclei to undergo fission without additional energy being put into the nucleus.
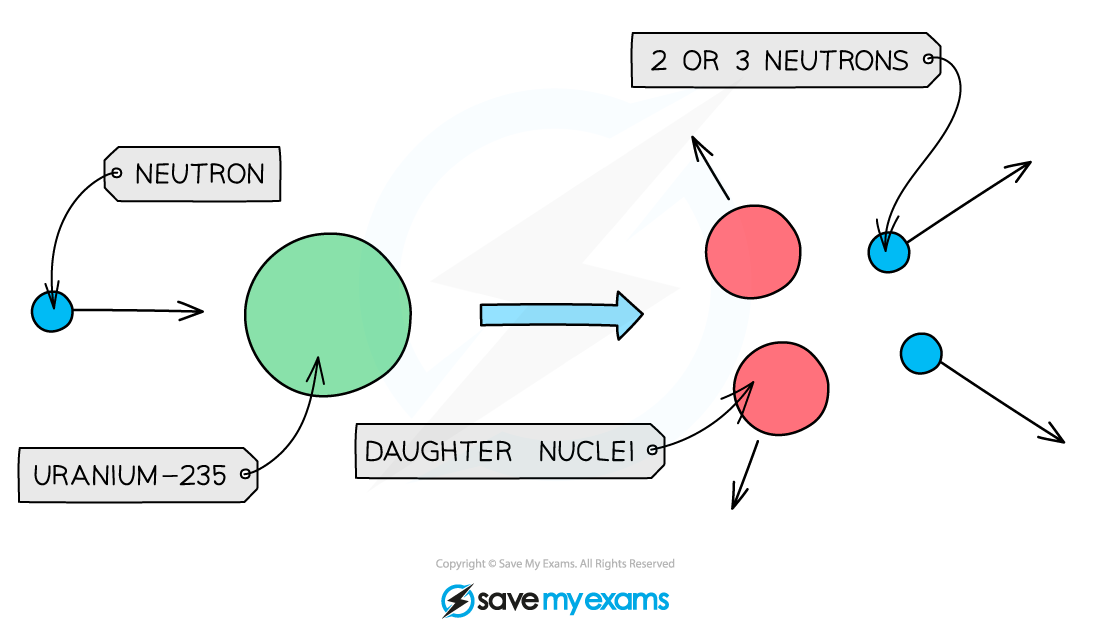
What is the structure of nuclear fission reactor?
Reactor core
A concrete-steel encasement around the core of the nuclear reactor.
Moderator
The purpose of a moderator: To slow down neutrons
Control Rods
Purpose of a control rod: To absorb neutrons
Coolant
The purpose of coolant: To remove the heat released by the fission reactions
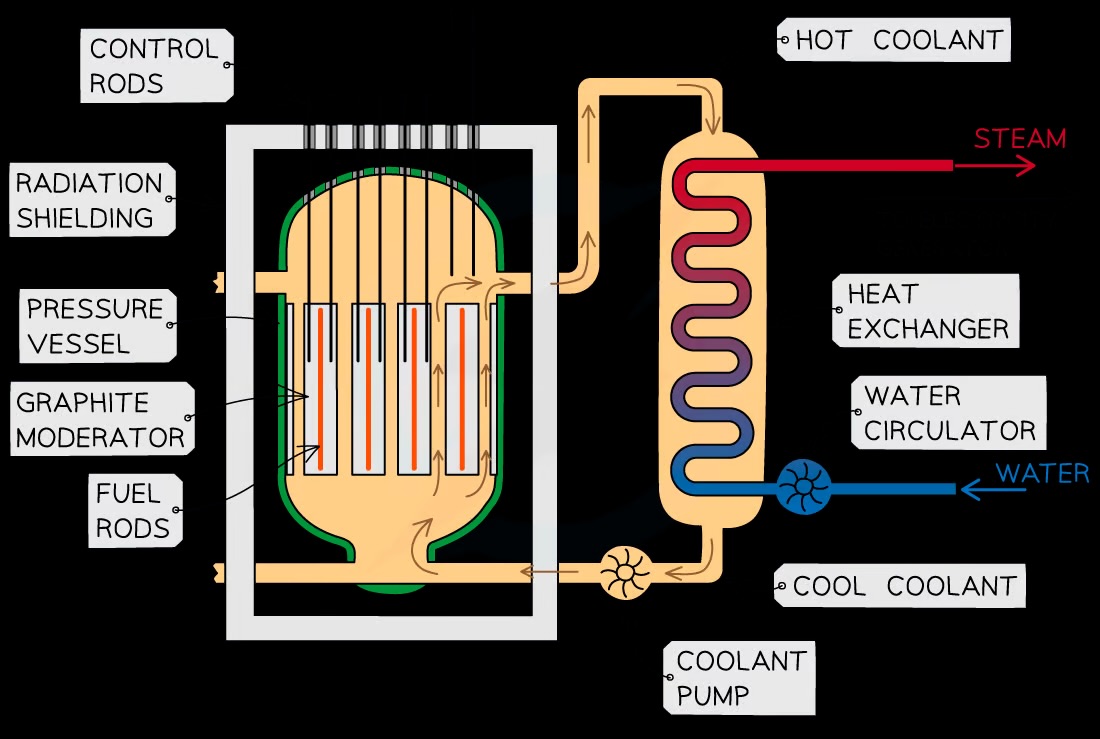
What is the function of the moderator?
The purpose of a moderator (made of graphite): To slow down neutrons
The moderator is a material that surrounds the fuel rods and control rods inside the reactor core
The fast-moving neutrons produced by the fission reactions slow down by colliding with the molecules of the moderator, causing them to lose some momentum
The neutrons are slowed down so that they are in thermal equilibrium with the moderator, hence the term ‘thermal neutron’
This ensures neutrons can react efficiently with the uranium fuel.
What’s the function of the control rod?
Purpose of a control rod ( made of boron steel): To absorb neutrons
The number of neutrons absorbed is controlled by varying the depth of the control rods in the fuel rods
Lowering the rods further decreases the rate of fission, as more neutrons are absorbed
Raising the rods increases the rate of fission, as fewer neutrons are absorbed
This is adjusted automatically so that exactly one fission neutron produced by each fission event goes on to cause another fission
In the event the nuclear reactor needs to shut down, the control rods can be lowered all the way so no reaction can take place
What’s the function of the coolant?
The purpose of coolant: To remove the heat released by the fission reactions
The coolant carries the heat to an external boiler to produce steam
This steam then goes on to power electricity-generating turbines
What is a low-level waste?
Low-level waste
This is waste such as clothing, gloves and tools which may be lightly contaminated
This type of waste will be radioactive for a few years, so must be encased in concrete and stored a few metres underground until it can be disposed of with regular waste
What is an intermediate- level waste?
Intermediate-level waste
This is everything between daily used items and the fuel rods themselves
Usually, this is the waste produced when a nuclear power station is decommissioned and taken apart
This waste will have a longer half-life than the low-level waste, so must be encased in cement in steel drums and stored securely underground
What is a high-level waste and how should it be treated?
High-level waste
This waste comprises of the unusable fission products from the fission of uranium-235 or from spent fuel rods
This is by far the most dangerous type of waste as it will remain radioactive for thousands of years
As well as being highly radioactive, the spent fuel roads are extremely hot and must be handled and stored much more carefully than the other types of waste
How high-level waste is treated:
The waste is initially placed in cooling ponds of water close to the reactor for a number of years
Isotopes of plutonium and uranium are harvested to be used again
Waste is mixed with molten glass and made solid (this is known as vitrification)
Then it is encased in containers made from steel, lead, or concrete
This type of waste must be stored very deep underground

What is nuclear fusion and its conditions?
when two smaller nuclei join together to form one larger nucleus.
The sum of the binding energy of smaller nuclei is smaller than of the larger nucleus formed.
The Coulomb repulsion must be overcome for the nuclei to come closer and form the larger nucleus.
High pressure
The temperature must be greater than or equal to 100MK.
For two nuclei to fuse, both nuclei must have high kinetic energy.
This is because the protons inside the nuclei are positively charged, which means that they repel one another.
It takes a great deal of energy to overcome the electrostatic force, so this is why it can only be achieved in an extremely high-energy environment, such as a star’s core.