C4 - Chemical Changes
1/55
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
What is an acid?
A substance that forms hydrogen ions (H⁺) when dissolved in water
Acids have a pH below 7
What is an alkali?
A soluble base that produces hydroxide ions (OH⁻) when dissolved in water
What is a base?
Any substance that reacts with an acid to form a salt and water
What is a neutralisation reaction?
The reaction between acids and bases
When hydrogen (H⁺) ions react with hydroxide ions (OH⁻) to form water
What is the equation for a neutralisation reaction?
Acid + base → salt + water
Why are acids neutralised in agriculture?
To improve the soil for crop growth
What does the pH scale measure?
How acidic or alkaline a solution is
How can pH be measured?
With a Universal Indicator or a pH probe
What is a strong acid?
An acid that is completely ionised in water. Particles dissociate to release H⁺ ions
What is a weak acid?
An acid that does not fully ionise in water.
Only a small proportion of particles dissociate to release H⁺ ions
What are some examples of strong acids?
Hydrochloric Acid (HCl), Nitric Acid (HNO₃), and Sulfuric acid (H₂SO₄)
What are some examples of weak acids?
Ethanoic acid, citric acid, and carbonic acid
Explain the difference between the strength and concentration of acids
The strength of an acid tells you the proportion of particles that ionise in water
The concentration of an acid measures how much acid there is in a given volume
What does pH measure?
The pH is a measure of the concentration of H⁺ ions in the solution
For every decrease of 1 on the pH scale, the concentration of H⁺ ions increases by a factor of 10
What do acids react with metal oxides to form?
Acid + metal oxide → salt + water
What do acids react with metal hydroxides to form?
Acid + metal hydroxide → salt + water
What is the equation for an acid and metal?
Acid + metal → salt + hydrogen
What do acids react with metal carbonate to form?
Acid + metal carbonate → salt + water + carbon dioxide
What do metals react with water to make?
Metal + water → metal hydroxide + hydrogen
What does the reactivity of a metal depend on?
How easily it loses its electrons to form positive ions
What is the reactivity series?
A list of metals arranged by their reactivity, from most reactice to least reactive
What is the order of the reactivity series
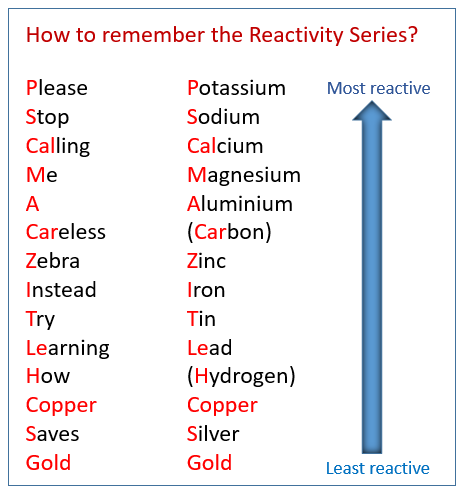
How can you tell the reactivity of a metal when they react with an acid?
The rate at which bubbles of hydrogen are given off
The more reactive the metal is, the faster the reaction will happen
What is a displacement reaction?
When a more reactive metal pushes a less reactive metal out of a compound
How are most metals found in nature?
As compounds in ores
How can metals be extracted from their oxides?
By reduction using carbon, or by electrolysis
Which metals can be extracted by reduction with carbon?
Metals that are less reactive than carbon
What metals can be extracted by electrolysis?
Metals that are more reactive than carbon
What is reduction in terms of oxygen?
Loss of oxygen
What is oxidation in terms of oxygen?
Gain of oxygen
What happens to a metal oxide when reduced by carbon?
The metal is produced, and carbon dioxide is formed
What happens to carbon in oxidation reactions?
Carbon is oxidised
What is oxidation in terms of electrons?
Loss of electrons
What is reduction in terms of electrons?
Gain of electrons
What is a redox reaction?
A reaction where reduction and oxidation happen at the same time
In a metal displacement reaction, what happens to the more reactive metal?
It is oxidised as it loses electrons
What happens to the less reactive metal in a displacement reaction?
It is reduced as it gains electrons
What is electrolysis?
Using electricity to break down a compound
What type of substance can be electrolysed?
Ionic substances when molten or dissolved in water
What is the positive electrode called?
Anode
What is the negative electrode called?
Cathode
Where do the positive ions (cations) go?
To the negative electrode (cathode)
Where do the negative ions (anions) go?
To the positive electrode (anode)
What happens to the positive ions (cations) at the cathode?
They gain electrons (reduced)
What happens to the negative ions (anions) at the anode?
They lose electrons (oxidised)
What products are formed at each electrode?
Metal at the cathode
Non-metal at the anode
Why are the electrodes made of graphite?
Conducts electricity (delocalised electrons which can carry the current)
Withstands high temperatures (strong covalent bonds), without breaking down
It’s inert, meaning it does not easily react with other substances
What happens when an ionic compound is molten?
The ions are free to move
What happens during electrolysis of molten lead bromide (PbBr₂)?
Metals form positive ions, so Lead is attracted to the negative electrode and gains electrons. Lead is formed at the cathode
Non-metals form negative ions, so Bromine is attracted to the positive electrode and loses electrons. Bromine gas forms at the anode
What additional ions are present in aqueous solutions
H⁺ ions and OH⁻ ions from the water
How is the product at the cathode decided?
If the metal is more reactive than hydrogen, hydrogen is produced
If the metal is less reactive than hydrogen, a solid layer of the pure metal will form
How is the product at the anode decided?
If OH⁻ and halide ions are present, molecules of chlorine, bromine, or iodine form.
If no halide ions are present, the OH⁻ ions are discharged, oxygen is produced
What are the half equations for water electrolysis?
At the cathode: 2H⁺ + 2e⁻ → H₂
At the anode: 4OH⁻ → O₂ + 2H₂O + 4e⁻.
Why are inert electrodes used?
To avoid them reacting with the products
How are soluble salts made?
By reacting acid with an excess insoluble base, then filtering and crystallising
Why is excess base used?
To make sure all the acid is neutralised