2.2 Membranes and transport
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
what are membranes made of
membranes are made of phospholipids and protiens
phospholipid structure
amphipathic
hydrophilic head of phosphate +glycerol
hydrophibic fatty acid tails (satured or unsaturated)
peripheral proteins
are attached to one surface of the bilayer
integral protiens
integral proteins can be polytopic or monotopic
are embedded into the membrane, many go all the way through and are polytopic so they can go through multiple surfaces. integral protiens going through one surface are monotopic
how did the davson danielli model come to be?
davson danielli proposed the model of the protien lipid sandwich
protiens that coat the outer surface of a phospholipid bilayer
this is because in an electron microgrpah membranes appeared as 2 dark parallel lines with a lighter region in between, appearing trilaminar and at that time we knew that protiens were dark and phospholipids light
outline 4 issues with the protien sandwhich model
assumes all membranes were of a uniform thickness and would have a constant lipid to protien ratio. not true because membranes serve different functions so their thickness and composition depend on their role
assumes all membranes have the same internal and external surfaces
it didnt account for the permeability of certain substances — didnt think about how there are hydrophilic pores needed so polar and charged molecules could enter
the temperatures which membranes became solid didnt correlate with those expected under the proposed model
what 2 discoveries disproved davson daneillis protein sandwich
membrane protiens are insoluble in water so there shouldnt be such a large and continous layer of hydrophilic protien around the outer surface of the membrane
fluorescent antibody tagging of membrane protiens showed that membrane protiens were mobile and not fixed so they arent in a static layer!
explain the fluid mosaic model/singer nicolson model
the fluid mosaic model describes the plasma membrane structure as a randomly organized fluid with a masaic of phospholipids, cholesterol, protiens and carbs that acts as a good barrier between aqueous solutions
explain why due to the amphipathic nature, phospholipids will naturally form bilayers
Phospholipids are amphipathic, possessing a polar, hydrophilic (water-loving) head and two nonpolar, hydrophobic (water-fearing) tails.
In an aqueous environment, the molecules spontaneously arrange into a bilayer because this configuration is the most energetically favorable:
The hydrophilic heads face outward, interacting favorably with the surrounding water.
The hydrophobic tails cluster inward, shielded from the water to form a nonpolar core.
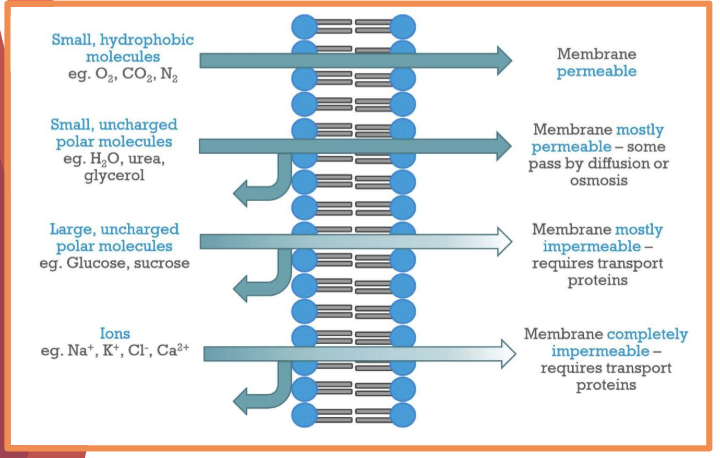
the hydrophobic fatty acid chains form the core of the membrane providing low permeability to____4
large molecules
hydrophilic particles
charged ions
polar molecules
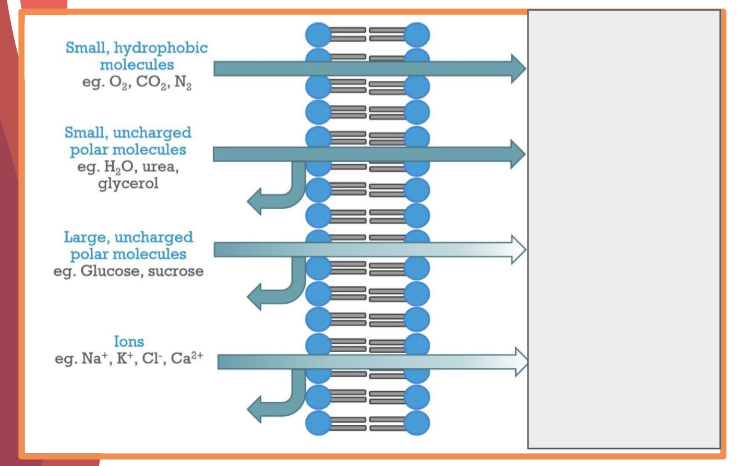
what is the permeability of the following
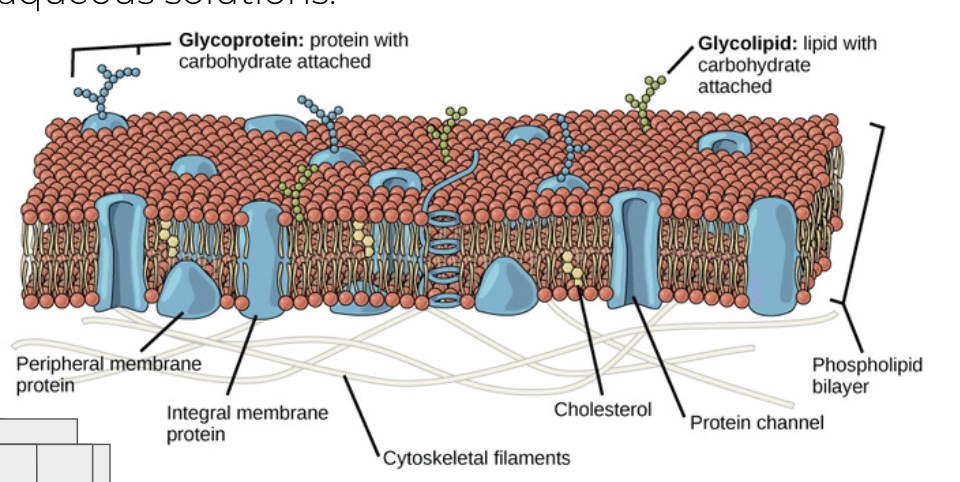
Transport: Protein channels (facilitated) and protein pumps (active)
Receptors: Peptide-based hormones (insulin, glucagon, etc.)
Anchorage: Cytoskeleton attachments and extracellular matrix
Cell recognition: major histocompatibility cells (MHC) proteins and antigens
Intercellular joinings: Tight junctions and plasmodesmata
Enzymatic activity: Metabolic pathways (e.g. electron transport chain)
oligosaccharide chain
short carbohydrate chain
how are glycoprotiens made
made when an oligosaccharide chain (multi sugar carb) is attatched to a protien via the process of glycosylation
function of a glycoprotien
the carb portion of a glycoprotien is located on the extracellular side (outside) of the membrane so it can do its job of cell reception, signalling and recognition
what is the purpose of cholesterol in the membrane
cholesterol helps adjust the fluidity of the membrane by stabilizing membranes at higher temperatures and preventing stiffening at lower temperatures
how is cholesterol positioned in the membrane
cholesterol is a sterol, so it has a 4 carbon ring structure and a OH hydroxyl group
the hydroxyl group makes the head polar and hydrophilic so its attracted to the heads of the phospholipids on the periphery of the membrane
the 4 carbon ring tail is hydrophobic so its attracted to the hydrophobic tails of phospholipids in the centre of the membrane
why is it important to regulate the degree of fluidity (3)
Membranes need to be fluid enough that the cell can move.
Membranes need to be fluid enough that the required substances can move across the membrane.
If too fluid however the membrane could not effectively restrict the movement of substances across itself.
what does membrane fluidity depend on?
the fatty acid composition (saturated vs unsaturated) of the lipid bilayer and the amount of cholesterol
why are saturated fatty acids less fluid in the membrane
saturated fatty acids are packed close together making the membrane less fluid and more structured. unsaturated fats have more kinks and are more flexible and fluid
cholesterols role in membrane fluidity
how does cholesterol influence membrane permeability for small hydrophilic molecules and ions (small polar and charged)
🧱 1. Cholesterol has two main effects on membranes
Cholesterol does two related but seemingly opposite things depending on the situation:
At moderate/high temperatures:
It reduces membrane fluidity by restraining the movement of phospholipids — this helps maintain membrane integrity (so the membrane doesn’t become too soft or leaky).At low temperatures:
It increases membrane fluidity by preventing phospholipid tails from packing too tightly together or crystallizing — this stops the membrane from becoming too rigid or solid.
⚖ 2. How it does both
Cholesterol’s structure explains this dual role:
It has a rigid, planar ring structure that fits between the fatty acid tails of phospholipids.
This restricts movement of the fatty acid chains (reducing fluidity at higher temps).
But at the same time, it disrupts tight packing (increasing fluidity at lower temps).
So cholesterol acts like a buffer — it keeps the membrane’s fluidity within an optimal range.
💧 3. Cholesterol and permeability
Cholesterol’s bulky, hydrophobic structure also reduces permeability to small water-soluble (hydrophilic) molecules and ions like Na⁺ or H⁺. That’s because it fills gaps between phospholipids, making it harder for polar molecules to slip through.
draw a cell membrane with all the components
phospholipid bilayer, hydrophic tail and hydrophilic heads
integral proteins
channel proteins
peripheral protiens
glycoprotiens + oligosacc. chain
cholesterol
glycolipid
name the 4 membrane functions
selectively permeable barrier — aka it controls the movement of particles across the membrane (not everything can go through)
water balance — controls the amount of osmosis that takes place
formation of vesicles or fusion of vesicles with the membrane for endo/exo cyctosis
cell-cell adhesion — there are these molecules called CAMs aka cell adhesion molecules that create cell to cell connections by binding one cell to another through an extracellular matrix component
explain the self sealing property of the bilayer
Because the hydrophobic tails avoid water, if the bilayer is disturbed (e.g., torn or pinched), the phospholipids spontaneously rearrange to minimize exposure of the tails to water.
👉 This means the membrane can bend, fuse, and reseal itself easily — a key feature for vesicle formation.
The cell membrane model proposed by Davson–Danielli was a phospholipid bilayer sandwiched between two layers of globular protein. Which evidence led to the acceptance of the Singer–Nicolson model?
A. The orientation of the hydrophilic phospholipid heads towards the proteins
B. The formation of a hydrophobic region on the surface of the membrane
C. The placement of integral and peripheral proteins in the membrane
D. The interactions due to amphipathic properties of phospholipids
C. The evidence supported that membranes have both integral and peripheral proteins, randomly distributed (forming a fluid mosaic), not a continuous protein layer.
the davson-danielli model assumed that proteins were only on the surface of the membrane, but they were actually integrated into the membrane
what are the 3 factors that affect whether a molecule can move through a membrane
size of particle
polarity
charge → biggest factor, no ions can pass through memrbanes
what are the two main types of membrane transport
passive transport, the cell does not need to expend any energy for the movement of molecules (the concentration gradient is down)
active transport: the cell is going up its concentration gradient, the cell needs to expend energy for the movement of molecules
explain passive transport and the types
passive transport is the movement of molecules that doesnt require energy from the cell. so molecules will move from areas of high concentration to areas of low concentration
types of passive transport:
simple diffusion
osmosis
facilitated diffusion
factors affecting the rate of diffusion
concentration gradient
temperature
SA:V ratio (aka the distance stuff has to diffuse)
size of particles
permeability of membrane
effect on rate of diffusion:
high concentration gradent
low concentration gradient
high SA
low SA
long diffusion path
short diffusion path
more permeable membrane
smaller particles
high concentration gradent — high rate of diffusion
low concentration gradient — low rate
high SA — high rate
low SA — slow
long diffusion path — slow
short diffusion path — fast
more permeable membrane — faster
smaller particles — faster (easier to pass through membrane and faster speed)
1.1: Smaller particle size increases the rate of diffusion.
1.2: Diffusion is the net movement of particles from a region of higher concentration to a region of lower concentration.
1.3: At the same temperature, particles have the same average kinetic energy; therefore, smaller particles have a greater velocity and move faster.
what adaptations are there that reduce the length of the diffusion path?
we can fold membranes to increase the SA:V ratio. this means theres more membrane in a smaller volume so substances will generally have to cross shorter distances across membranes = shorter diffusion distance
what are some adaptations to maximizing the surface area for absorption
a lot of it is jsut folding the membrane more like the cristae in the mitochondria of thylakoid in chloroplasts
difference between diffusion and osmosis
KEY: osmosis may occur when there is a partially permeable membrane like a cell membrane
diffusion is the movement of molecules from high concentration to low concentration, here both the solute and solvent move
osmosis is the movement of solvent only across a semipermeable membrane from high to low solvent concentration (low solute to high solute concentration)
what are aquaporins?
an aquaporin is an integral protien that acts as a pore in the membrane that functions to speed up the movement of water molecule
hypertonic solution
the solution has a higher concentration of solute
isotonic
the solution is equal in concentration of solute
hypotonic
the solution has a lower concentration of solute
plasmolyzed plant cells
solvent concentration aka water concentration is low outside the cell so the water leaves the plant cell vacuole leaving the plant cell plasmolyzed
flaccid plant cell
this occurs when there are equal concentrations of water outside and inside (isotonic) so water does in and out in equal amounts
turgid plant cells
high water concentration outside the cell, lower water concentration inside of cell, so water comes in and expands the vacuole and causes the cell to be turgid.
what is facilitated diffusion
its the movement of particles along a concentration gradient (high to low concentration) but using a protien
these protiens can be channel or carrier protiens
draw a channel and carrier protien
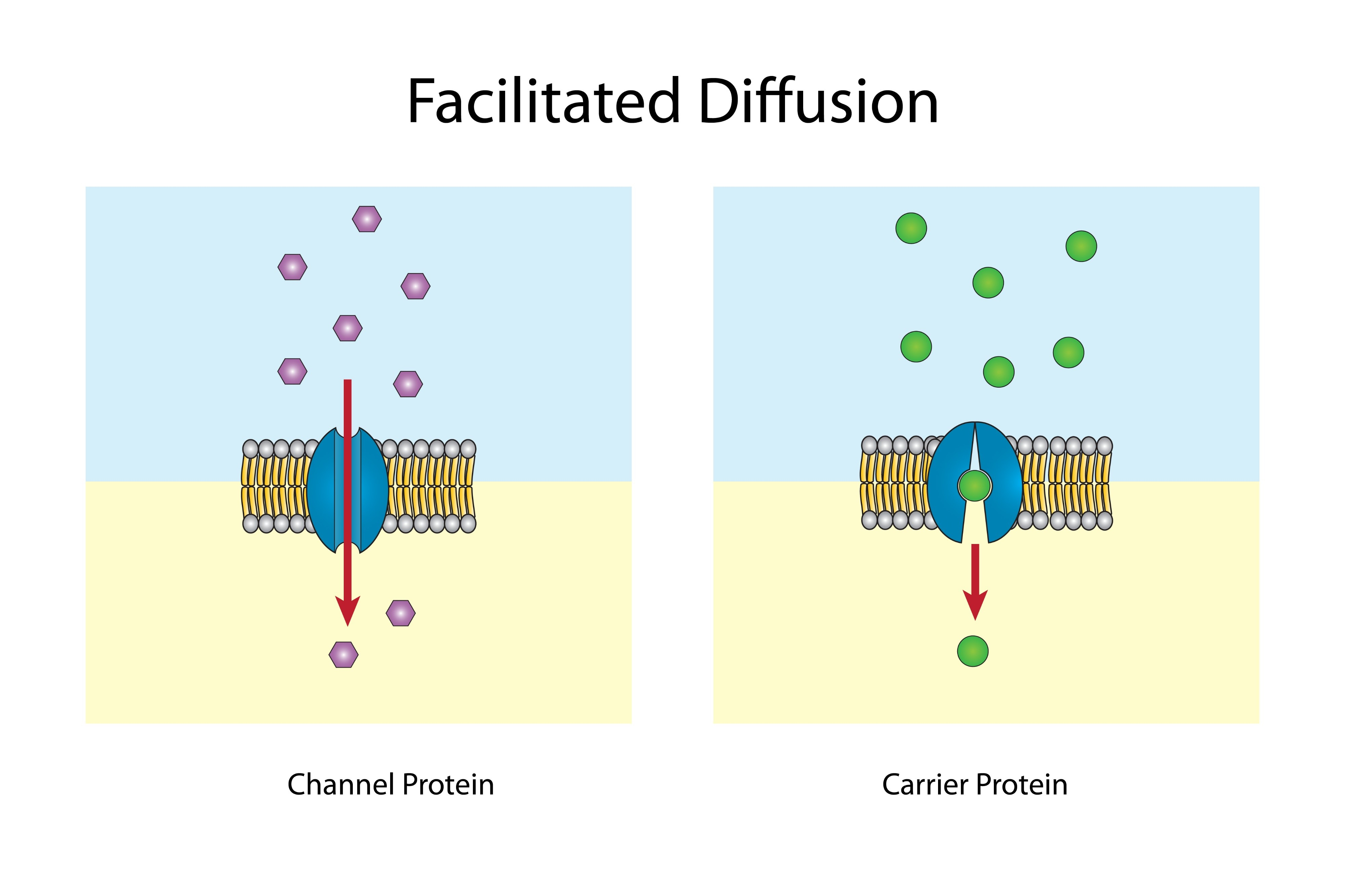
what is a channel protien
an integral, polytopic protien channel that opens through external stimuli. when open, particles can flow through freely from high to low concentration.
what is a carrier protien
an integral, polytopic protien that relies on molecules binding to itself in order to induce a conformational change that physically moves the particle across the membrane, releasing it on the other side.
what is active transport
active transport requires the cell to use energy (ATP). the reason being that molecules need to flow from areas of low concentration to areas of high concentration
basically ways to transporting molecules against their concentration gradient
2 types of active transport
primary active transport (uses ATP) — protien pumpts, endocytosis, exocytosis
secondary active transport aka co-transport
what are protein pumps
these are integral protiens that require ATP to undergo shape changes (allosteric) changes
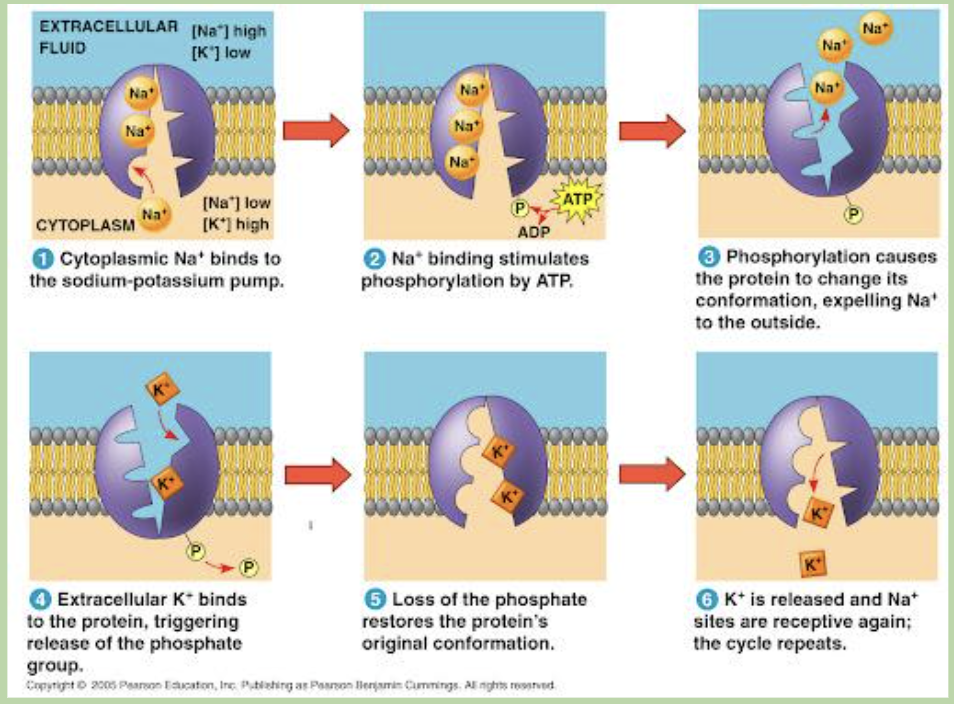
an Na-K pump is an example of what kind of transporter?
Na-K pump is an example of exchange transporter
outline the process of an Na-K pump (6)
comment on the flow of Na and K, how is the flow an example of active transport?
Na flows from cytoplasm to extracellular fluid
K flows from extracellular fluid to cytoplasm
their directions are from low to high concentration
endocytosis
endocytocis is the process by which cells actively engulf and internalize materials from their external environment by folding the plasma membrane inward forming a membrane bound vesicle. forming a vesicle takes ATP
types:
phagocytosis: takes in large solid particles, non specific
pinocytosis: the plasma membrane pinches inwards non selectively to wrap around the surrounding fluid
receptor mediated endocytosis: only wants specific macromolecules. target molecules bind to specific receptor proteins and once we know its what we want, it pinches off to form a vesicle
specialized Fd channels
these are an example of facilitated diffusion
these channel protiens are voltage gated so they open and close depending on the voltage of their environment.
when the cell has negative potential, the channel is closed. but once the inside it positive, the channel opens to let the K+ out.
either way, even though the channel protiens are gated (not always open) the Na and K flow from high to low concentrations, hence passive
exocytosis
uses atp to form vesicles that eject molecules. 2 types:
constitutive secretion — makes a vesicle and excretes molecules continuously
regulated secretion — makes vesicle, but waits for a trigger to excrete molecules
what are vesicles
vesicles are small sphereical packages that bud off the RER and the golgi apparatus, they carry protiens produced by ribosomes in the RER to the golgi apparatus where they are prepared for export from the cell via another vesicle
secondary active transport aka co-transport
so the second solute has a concentration gradient that was created through primary active transport.
the second solute diffuses across the membrane and this drives secondary active transport
where is the energy required for secondary active transport gained
the energy required for secondary active transport is derived from energy stored in the form of concentration differences in a second solute
in Na-K pump how many Na pumped out, how many K in?
3 Na, 2K
explain the Na-glucose secondary transport system
an electrochemical gradient is created by primary active transport and this gradient can move other solutes against their concentration gradients in a process called co transport
so in the Na-K pump, it used atp to power the pump and push Na against its concentration gradient to create a further electrochemical gradient. now Na flows down its concentration gradient through a Na-glucose cotransporter which transports glucose alongside Na. here glucose is going against its concentration gradient.
this is an example of symport, 2 molecules in same direction being transported togehter!
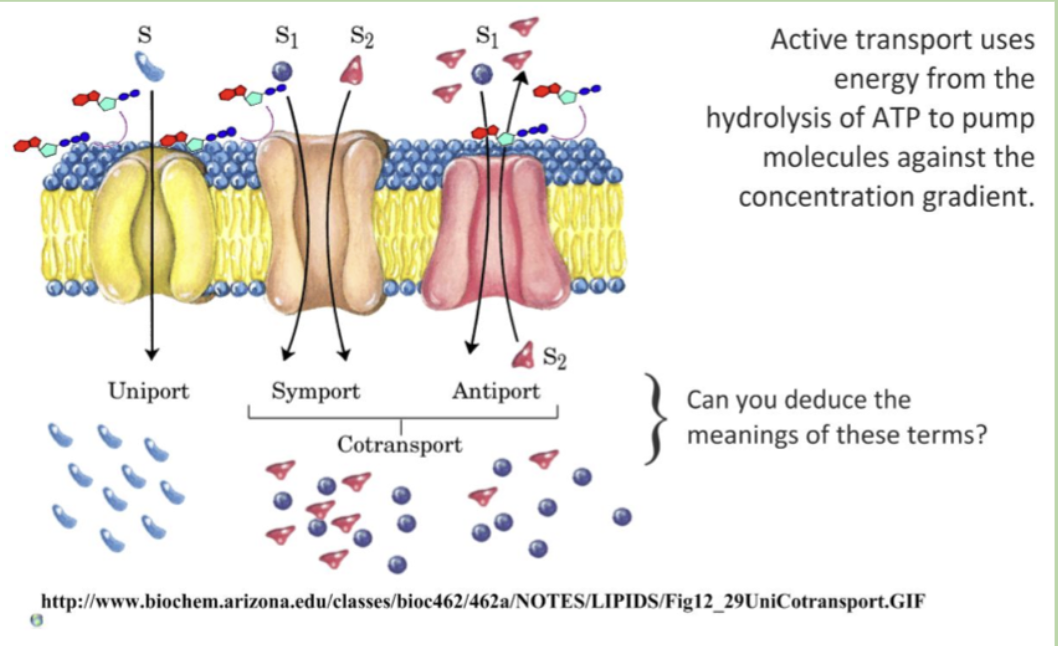
uniport
symport
antiport
uniport — transports 1 molecule in 1 direction
symport — transports 2 molecules in 1 direction
antiport — transports 2 molecules in opposite directions
only symport and antiport are cotransports because they involve the simultaneous movement of multiple molecules
all of these are channel protiens!
the Na-K pump is what type of co-transport?
antiport, molecules moving in different directions