science- chemical reactions, yr7
1/26
Earn XP
Description and Tags
all key terms and definitions from the year 7 topic chemical reactions
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms

what is this hazard symbol
flammable
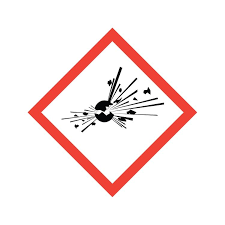
what is this hazard symbol
explosive

what is this hazard symbol
toxic

what is this hazard symbol
harmful to environment
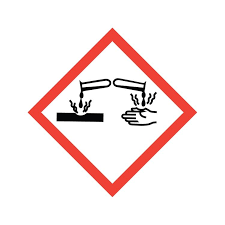
what is this hazard symbol
corrosive

what is this hazard symbol
irritant

what is this hazard symbol
hazard to health
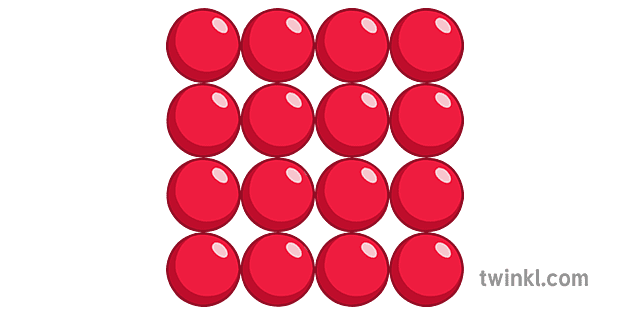
what is this a diagram of?
solid
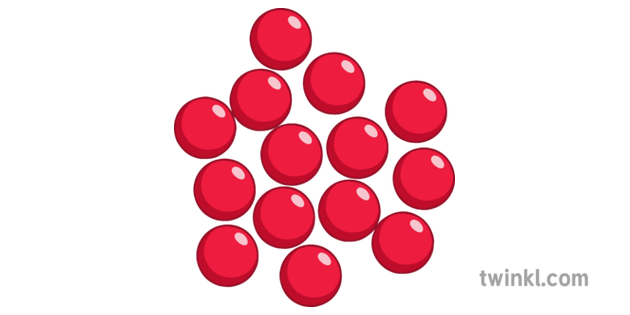
what is this a diagram of?
liquid
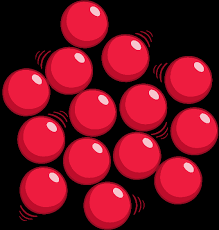
what is this a diagram of?
gas
physical change
the type of particle stays the same
chemical change
the type of particle changes
what do you use to measure the pH of something
universal indicator
on the pH scale, what colour is acid
red, 0-6
on the pH scale, what colour is alkali
purple, 8-14
on the pH scale, what colour is neutral
green, 7
neutralisation
a chemical reaction when acids and alkalis cancel the effects of each other out
acid+ alkali>
salt+ water
word equation
reactants> products
name 3 common laboratory strong acids
sulphuric acid, nitric acid, hudrochloric acid
metal + acid>
salt+ hydrogen
name 3 salts
magnesium sulphate, zinc chloride, iron nitrate
metal + hydrochloric acid>
metal chloride + hydrogen
metal + sulphuric acid>
metal sulphate + hydrogen
metal + nitric acid>
metal nitrate+ hyrdrogen
when metal carbonates react with dilute acids, they produce what 3 products?
carbon dioxide, a salt, water
acid+ carbonate>
salt+ carbon dioxide + water