Notebook LM: Lecture 6 Ch.8 Enzyme Mechanisms and Inhibitors
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
What does the term catalysis by approximation mean?
A strategy where the enzyme brings two substrates into close proximity and correct orientation on a single binding surface
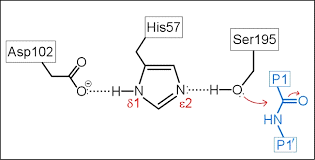
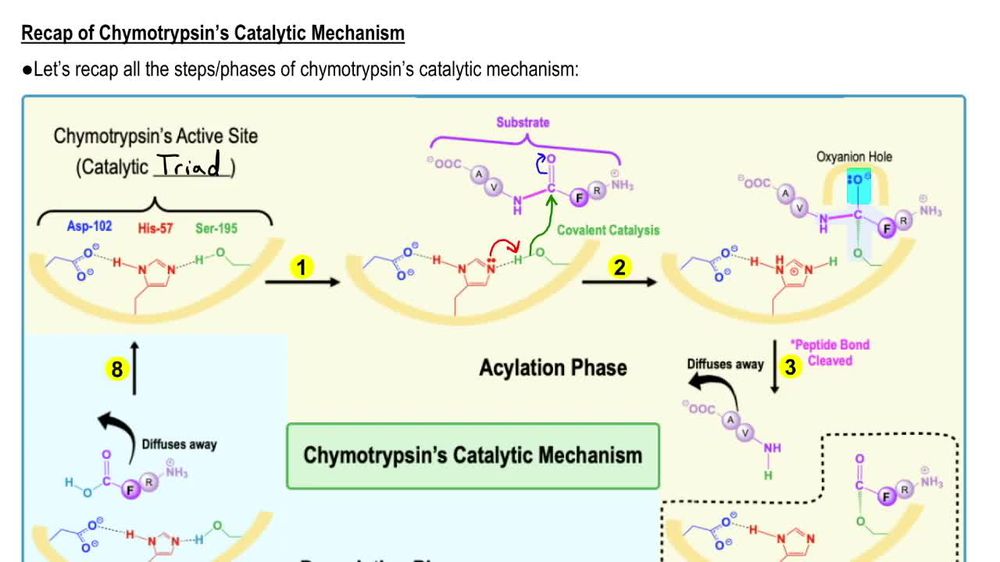
What is a catalytic triad?
A specific set of 3 residues (Asp 102, His 57, SER 195 in chymotrypsin) that function together to enhance the reactivity of a nucleophile
What is chymotrypsin?
A digestive enzyme (serine protease) that cleaves peptide bonds on the carboxyl side of aromatic or large hydrophobic
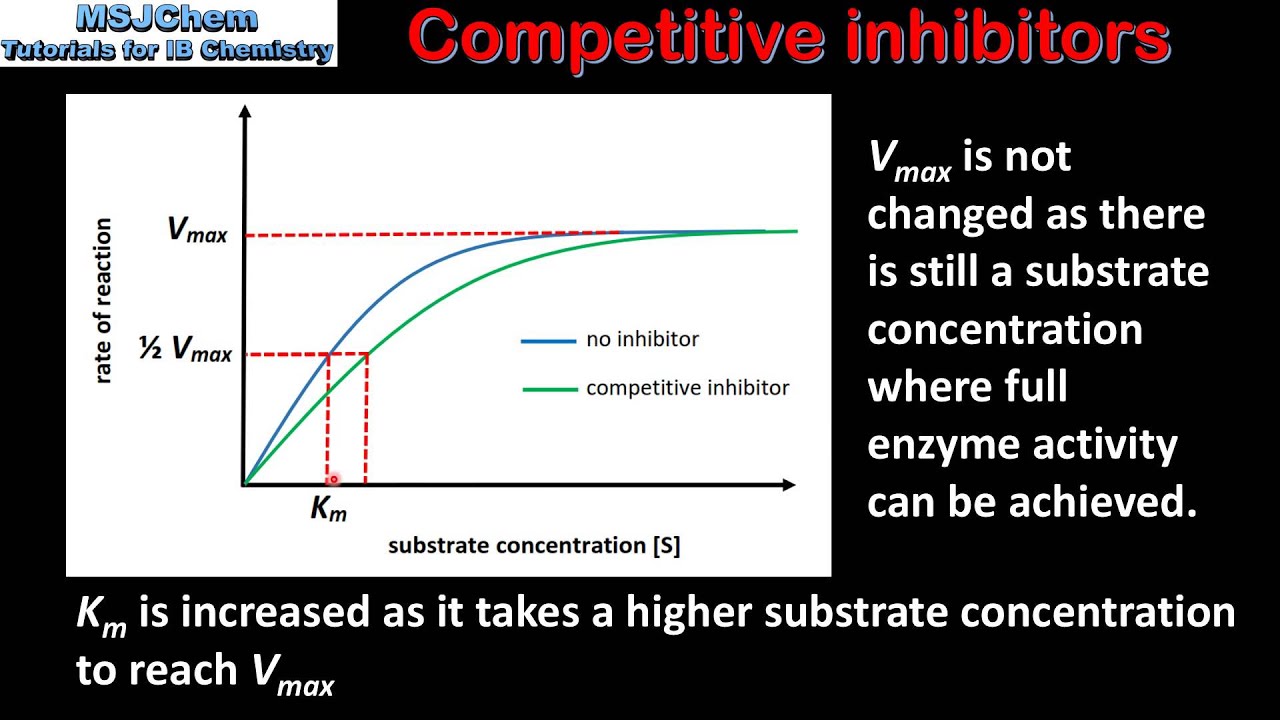
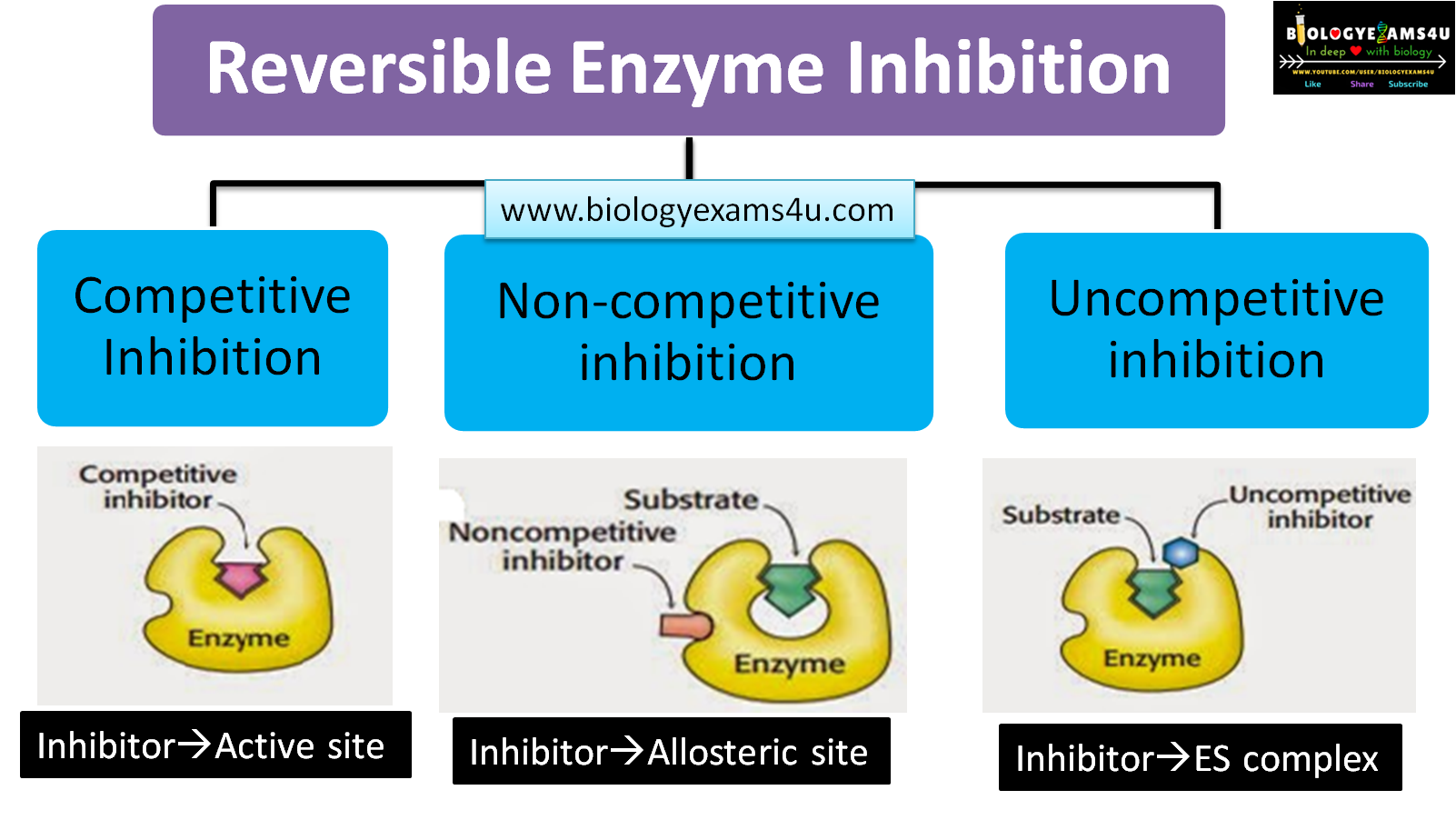
What is a competitive inhibitor?
A molecule that resembles the substrate and binds to the active site, preventing the substrate from binding
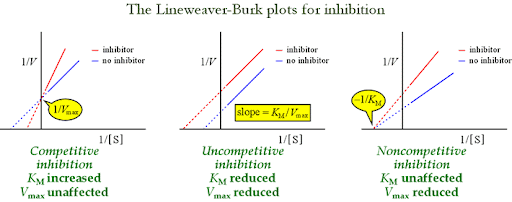
A competitive inhibitor _______ the apparent Km (lowers affinity) but does _____ ______ the Vmax, as high substrate concentrate can outcompete the inhibitor
Increases; does not
What is denaturation?
The loss of an enzymes native secondary and tertiary structure, usually due to extremes in temperatures or pH, resulting is loss of activity
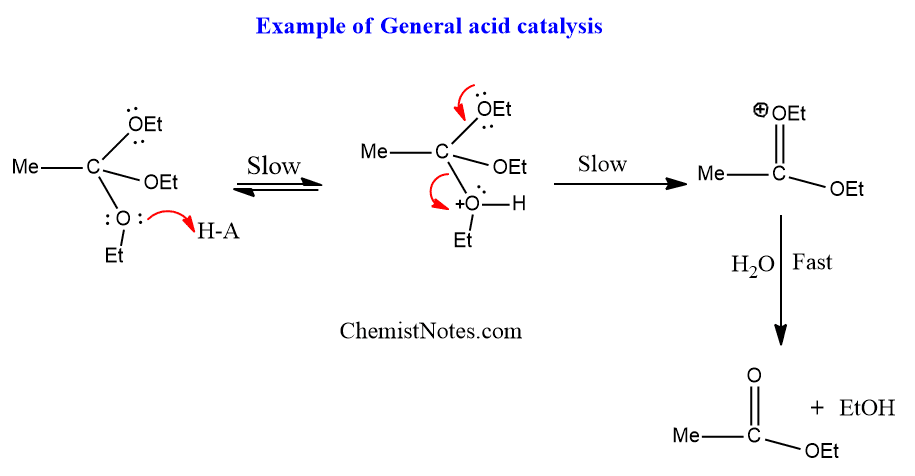
What is a general acid-base catalysis?
A mechanisms where a residue in the active site (other than water) acts as a proton donor or acceptor
What is Km (apparent)?
The concentration of substrate at which an enzyme yields half its Vmax in the presence of an inhibitor under non-ideal conditions
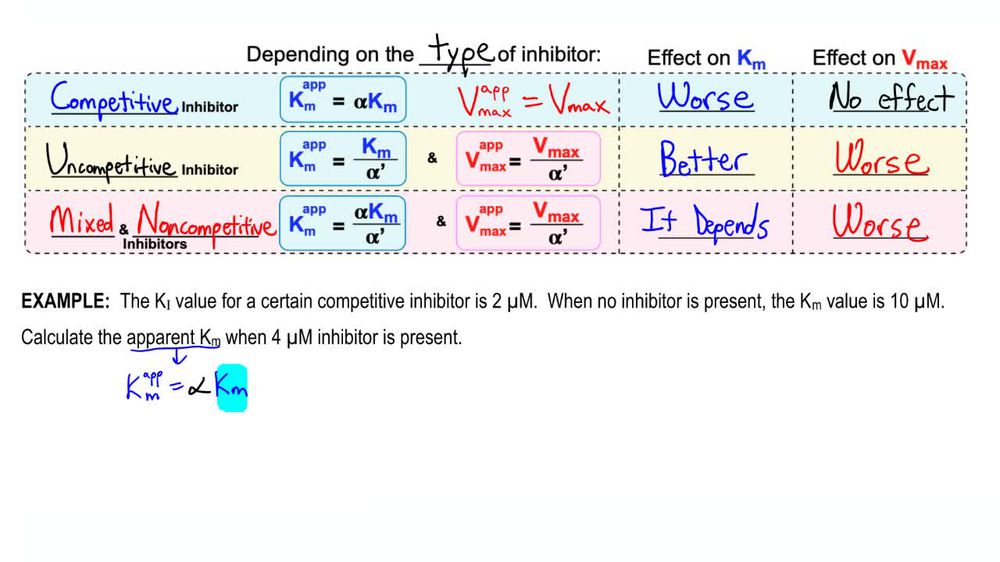
How does apparent Km differ from true Km?
Differs from true Km because it reflects effective affinity rather than the actual affinity, often increasing in the presence of competitive inhibitors
What is Near Attack Conformation (NAC)?
A specific precursor conformation of the enzyme-substrate complex that is positioned to move directly into the transition state
What is a noncompetitive inhibitor?
An inhibitor that binds to a site other than the active site, decreasing Vmax without affecting Km
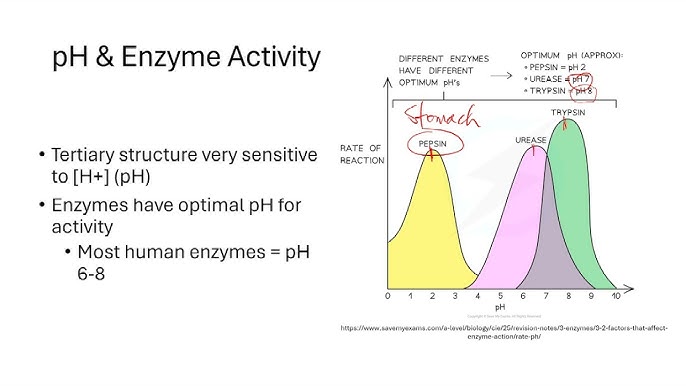
What is pH optima?
The specific pH at which an enzyme exhibits maximum activity, determined by the protonated states of its active site residues
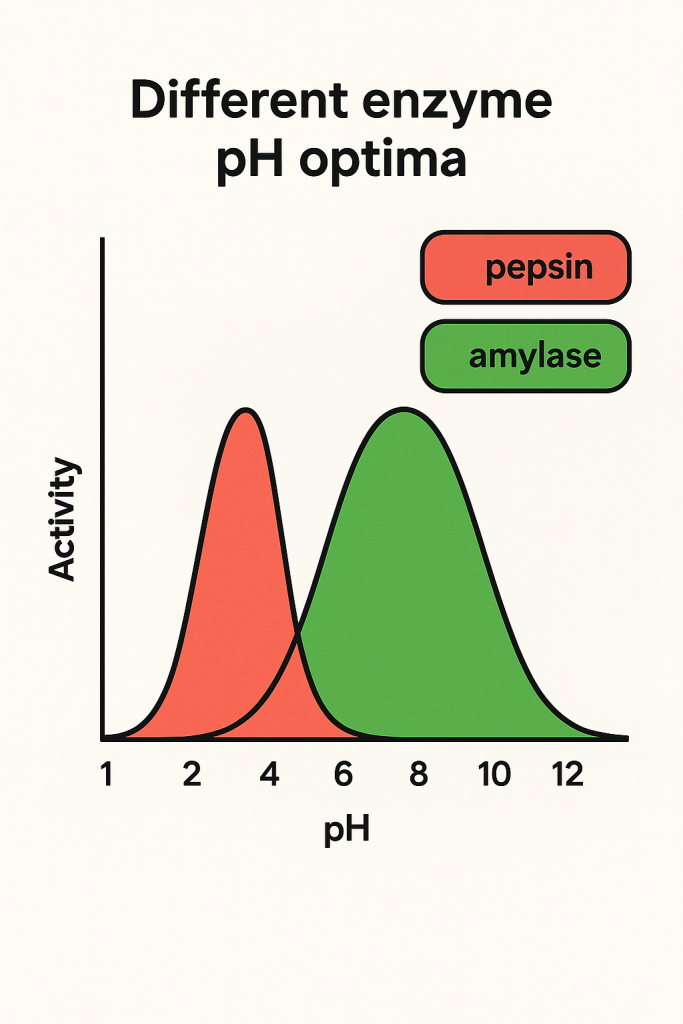
What is preorganization within an enzyme?
The structural arrangement of an enzymes active site during protein folding that allows it to stabilize a transition state with minimal entropic costs
What is a reversible inhibitor?
A substance that binds to an enzyme via non-covalent interactions and can be dissociated from the enzyme. It temporarily binds to the enzyme, slowing its activity w/o permanently damaging it, allowing the enzyme to function normally again once inhibitor is removed by weak noncovalent bonds
What is the Transition State Analog (TSA)?
A stable molecules that resembles the transition state of a reaction and binds to the enzyme active site with very high affinity
What is a uncompetitive inhibitor?
An inhibitor that binds only to the enzyme-substrate (ES) complex, preventing the reaction from completing
What is a zymogen?
An inactive precursor of an enzyme that requires proteolytic cleavage for activation (Ex: chymotrypsinogen)
How does temperature influence enzyme activity, and what occurs when temperature increases beyond a certain point?
Increasing temperature generally increases the rate of a reaction by increasing the frequency of interactions between the enzyme and substrate. However, further increases lead to denaturation, which is the disruption of the enzyme’s secondary and tertiary structures, rendering it inactive.
Explain why the protonation state of amino acid side chains is critical for the pH modulation of enzymes.
Many amino acids have ionizable side chains whose protonation sate varies with pH, which influences their ability to participate in catalysis or binding. Consequently, enzymes typically display a “pH optima”, a specific range where the active site residues are in the correct protonation state to function.
Describe the specific pH requirements for the activation of Triose Phosphate Isomerases (TPI)?
TPI requires two active site residues, Glu165 and His95, to be unprotonated to be active. Because Glu165 has a pKa of 4.0 and His has a pKa of 6.5, the enzyme only reaches its active state at a pH optimum greater than 6.5.
Define covalent catalysis and provide an example of an enzymes active site that utilizes this strategy.
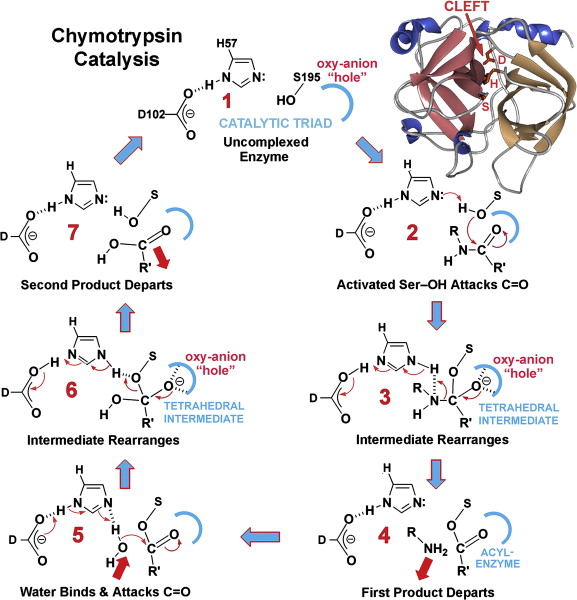
Covalent catalysis occurs when a residue in the enzyme’s active site undergoes a transitory covalent modification during the reaction. Chymotrypsin is a primary example, as it forms a transitory acylated intermediate with the substrate.
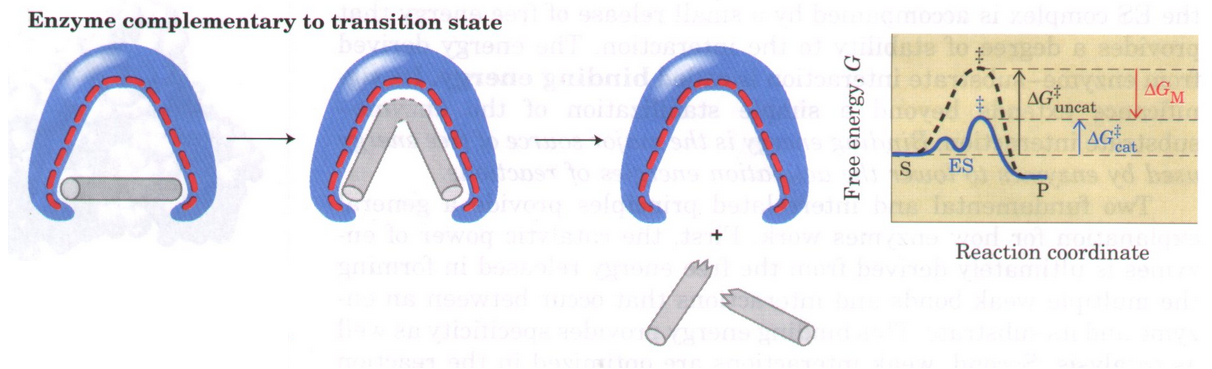
What is the “entropic cost” mentioned in the context of non-catalyzed reactions in water, and how do enzymes address this?
In water, reactants develop charges in the transition state that require water molecules to reorganize, which is entropically unfavorable (-S). Enzymes are “preorganized” to provide a low-dielectric environment that stabilizes these charges, paying the energetic cost during protein folding rather than the reaction
How does the mutation of Arginine 90 (Arg90) to Citrulline (Cit) affect the catalytic rate of Chorismate Mutase?
Replacing the positively charged Arginine with the uncharged Citrulline maintain the same number of hydrogen bonds but removes the electroststic stabilization of the transition state. This single change reduces the catalytic rate (Kcat) by a factor of 20,000
Distinguish between the “burst phase” and the “steady-state phase” in chymotrypsin kinetics.
The burst phase is a rapid initial step where a product (such as p-nitrophenol from a faux substrate) is quickly released as the enzyme acylated. The steady-state phase is a slower second state characterized by the turnover of the enzyme as it is de acylated to process more substrate.
How does a competitive inhibitor affect the Vmax and Km of an enzymatic reaction?
A competitive inhibitor has no effect on the Vmax because high concentrations of substrate can “wash out” the inhibitor. However, it increases the apparent Km, meaning more substrate is requires to reach half-maximum velocity
What is the mechanism by which Remdesivir inhibits the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp)?
Remdesivir is a nucleotide analogue of ATP that competes with ATP for incorporation into the RNA chain by RdRp. Once incorporated at position i, it allows synthesis to continue briefly before causing RnA synthesis termination at position i+3
What are the 4 steps within the mechanism of action within the catalytic triad?
Hydrogen bonding network
Proton transfer
Electrostatic stabilization
Nucleophile formation
Describe the role of Aspartate 102 in the chymotrypsin mechanism
Aspartate is negatively charged and uses its electrostatic influence to orient Histidine 57 and stabilize the positive charge that Histidine requires after it accepts the protein from serine
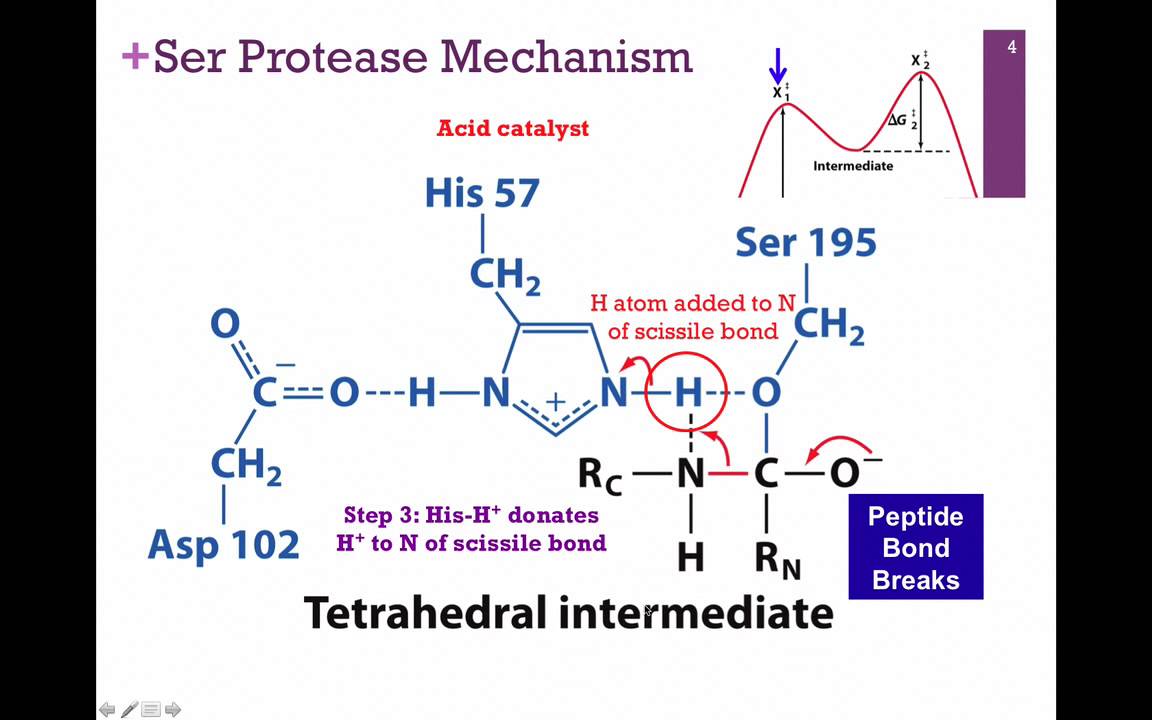
Describe the role of Histidine 57 in the chymotrypsin mechanism
This residue serves as a general base. Its role is to extract the proton from serine 195 hydroxyl group, which transforms the serine into a highly reactive alkoxide ion
Describe the role of Serine 195 in the chymotrypsin mechanism
This residue contains the primary hydroxyl group that acts as the nucleophile to attack the substrate. Under normal physiological conditions, a primary hydroxyl group group is a poor nucleophile because its pKa is greater than 14, meaning it is unlikely to lose a proton and become active.
What occurs during the electrostatic stabilization phase during the chymotrypsin mechanism?
As Histidine 57 becomes positively charged by taking the proton from serine 195 hydroxyl group, Aspartate 102 stabilizes the protonated Histidine through complementary electrostatic interactions
What occurs during the proton transfer phase during the chymotrypsin mechanism?
Histidine 57 pulls the proton away from the Serine 195 hydroxyl group
What occurs during the nucleophile formation phase during the chymotrypsin mechanism?
This coordinated action effectively lowers the pKa of the Serine hydroxyl group, allowing it to become a deprotanated alkoxide ion. In this state, Serine 195 is a good nucleophile and can perform the initial nucleophilic attack on the substrate, leading to the formation os a transitory tetrahedral intermediate and a subsequent acylated-enzyme intermediate.