ELECTRODE POTENTIALS AND CELLS
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
63 Terms
ELECTROCHEMICAL CELLS can be made from
2 different metals dipped in salt solutions of their own ions and connected by a wire (AKA the external circuit)
there are 2 reactions within an electrochemical cell
oxidation and reduction- this process is a REDOX
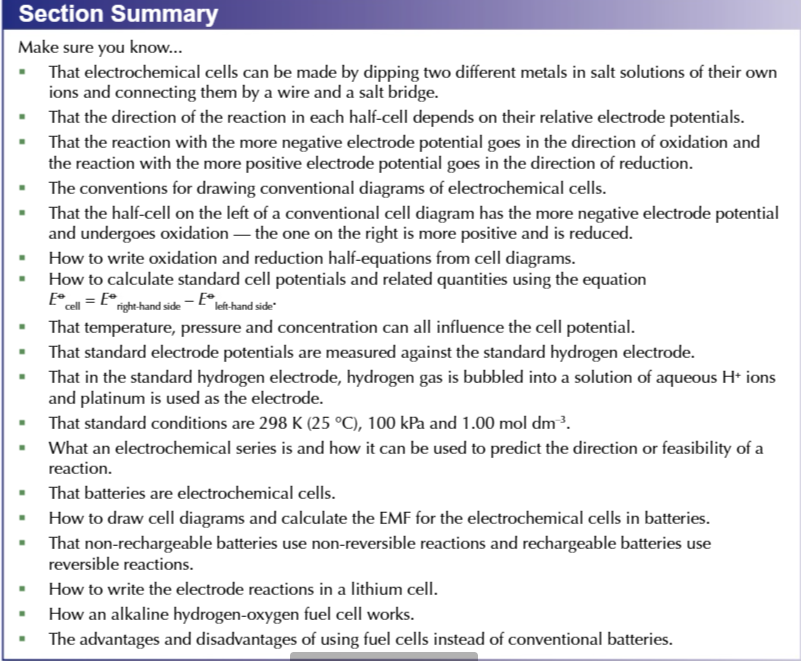
an electrochemical cell made of copper and zinc
zinc loses electrons more easily than copper, so at the ZINC electrode there is OXIDATION to form Zn2+
electrons from the zinc electrode are released into the external circuit into the copper electrode
reducing the Cu2+ ions into Copper atoms
electrons flow though the wire FROM the most reactive metal TO the least
the voltage between two half-cells can be calculated using a voltmeter to find the CELL POTENTIAL/ EMF
cathode charge
negative
anode charge
positive
reaction at cathode
reduction
reaction at anode
oxidation
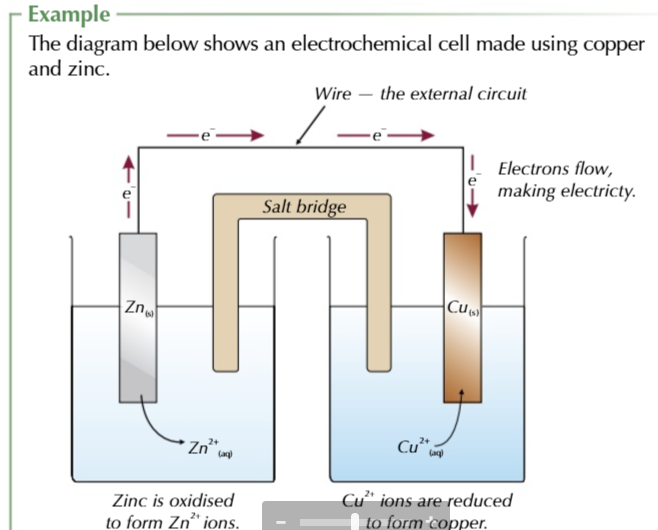
you can have half cells involving solutions of 2 aqueous ions of the same element
for example using Fe2+ and Fe3+
platinum is used because
its inert and conducts electricity
the conversion of Fe2+ to Fe3+
occurs on the surface of the platinum electrode
the direction of the conversion of 2+ to 3+ depends on the other half-cell in the circuit
if it contains a metal LESS reactive than iron, Fe2+ will be oxidised to Fe3+
if the other cell contains MORE reactive metal fe3+ will be reduced to fe2+ at the electrode

the direction of reaction depends on the metal able to lose electrons more easily (HOW EASILY OXIDISED)
How easily a metal oxidises is measured using electrode potentials
easily oxidised metals have VERY NEGATIVE potentials
easily reduced metals
have less negative/ positive electrode potentials
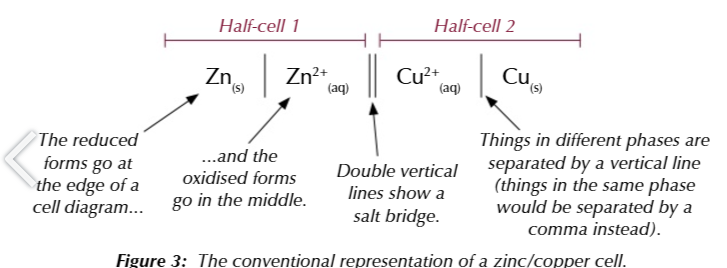
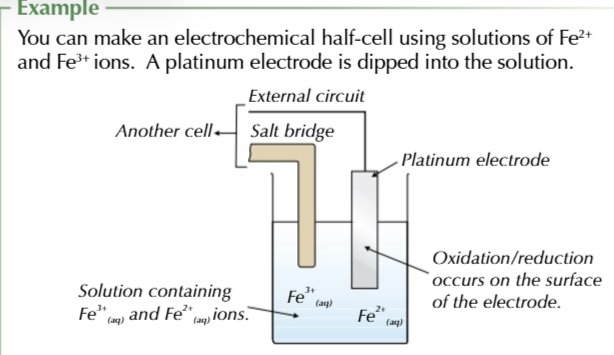
MORE NEGATIVE FIRST MORE POSITIVE SECOND


cell potential is ALWAYS positive
more positive - more negative

factors affecting the electrode potential
temperature 25℃
pressure 1atm
concentration 1 mol dm-3
changing the equilibrium position
changes the cell potential
standard conditions used to measure electrode potentials
so you get the same value for the electrode potential and can compare values for different cells
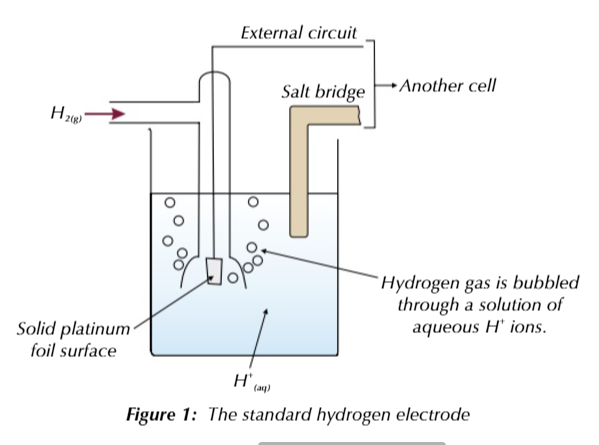
THE STANDARD HYDROGEN ELECTRODE
you measure the electrode potential of a half cell against the standard hydrogen electrode
in the standard hydrogen electrode, hydrogen gas bubbled through a solution of aqueous H+ ions
a platinum electrode is used as a platform for the oxidation/reduction reactions
standard conditions when measuring electrode potentials using SHE
all solution ceoncentrations 1.00 mol md-3
298K
100kPa
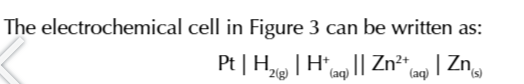
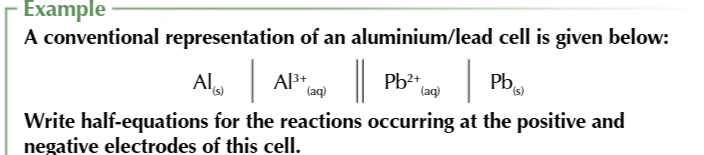
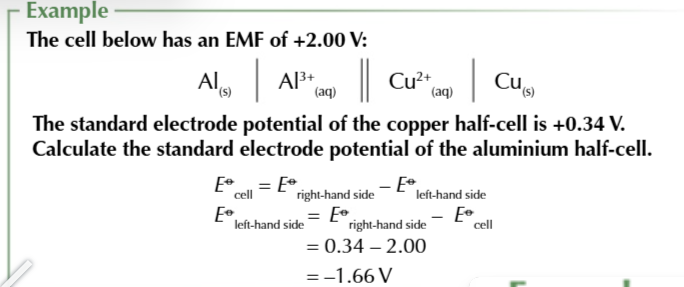
Standard electrode potential of a half cell is the voltage measured under standard conditions when
the half cell is connected to s SHE
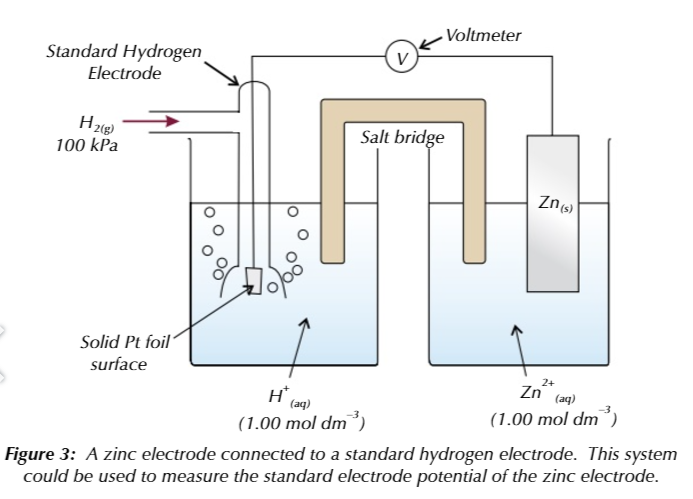
SHE is always on the left
no matter of which half cell is more positive

SHE can be used to calculate standard electrode potentials because
the electrode potential of a SHE is 0.00V therefore the voltage will be equal to the electrode potential on the RIGHT
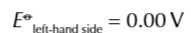
electrochemical series
long list of electrode potentials for different electrochemical half cells in order
half cells are always written as reduction reactions (GAIN ELECTRONS)
but reactions are reversible and can go the opposite way
when two half equations are put together in an electrochemical cell
the one with the more negative electrode potential goes in the direction of oxidation BACKWARDS
the more positive electrode potential goes in the direction of reduction FORWARDS
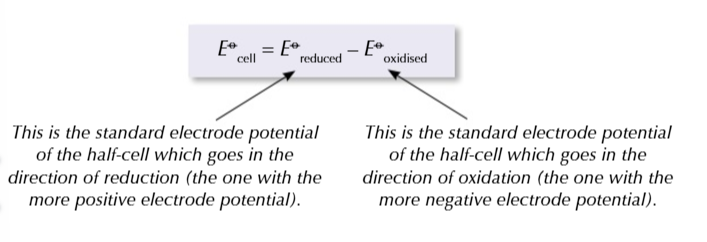

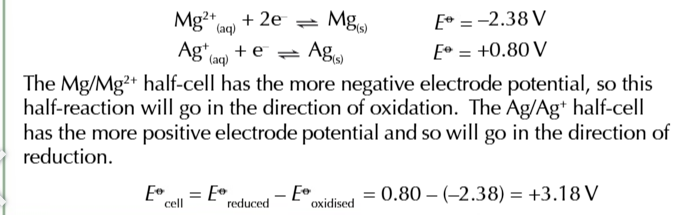
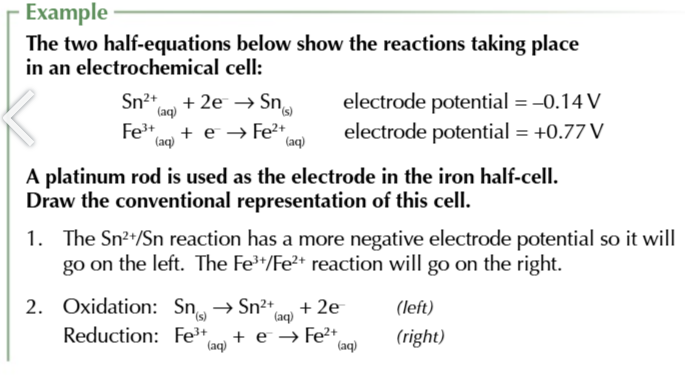
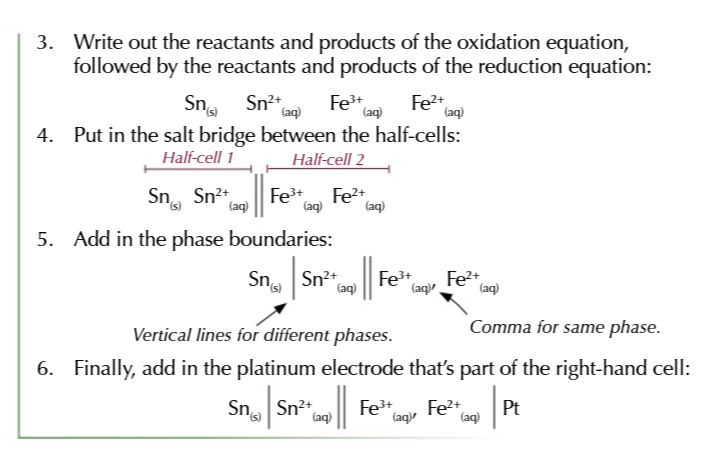
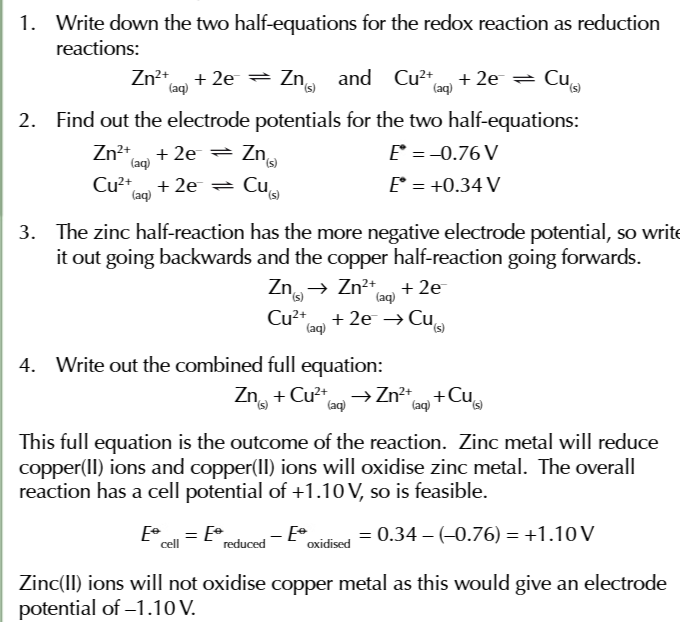
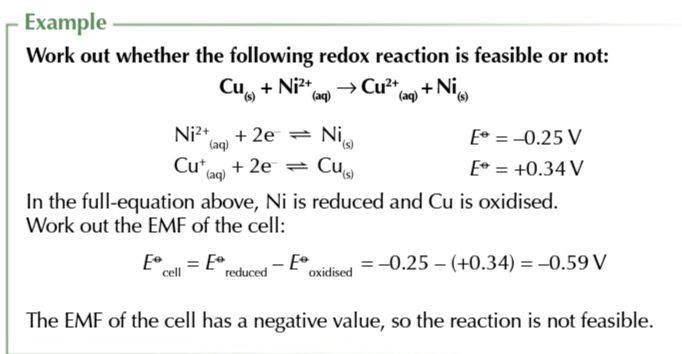
you can use electrode potentials to predict whether a redox reaction will occur and show which direction it goes in
find the 2 half equations for redox reactions and write them BOTH as reduction reactions (GAIN ELECTRONS)
Use the electrochemical series to work out which half equation has more negative electrode potential
write out the half equation with the more negative potential going in the backwards direction OXIDATION and the more positive potential going forwards REDUCTION
combine the 2 half equations and write out a full redox equation
when the overall electrode potential is positive
the reaction is feasible in that direction
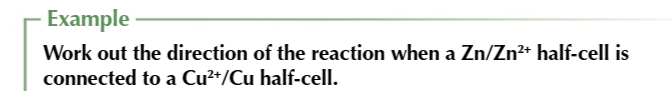

batteries types
rechargeable
non rechargeable
non rechargeable cells
use irreversible reactions
common type of non rechargeable cells
dry cell alkaline battery
short use gadgets that dont require alot of power use dry cell alkaline batteries:
TV remotes
smoke alarms
torch
Zinc-Carbon dry cells batteries
negative electrode: zinc
Positive electrode: Manganese dioxide and carbon mixture
Electrolyte: Ammonium chloride paste
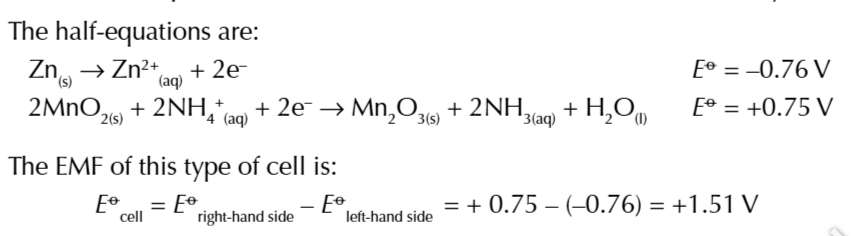
zinc electrode forms the casting of the battery which becomes thinner as the zinc is oxidised
which is why overtime batteries leak
devices with rechargeable batteries:
mobile phones
laptops
cars
common type of rechargeable battery
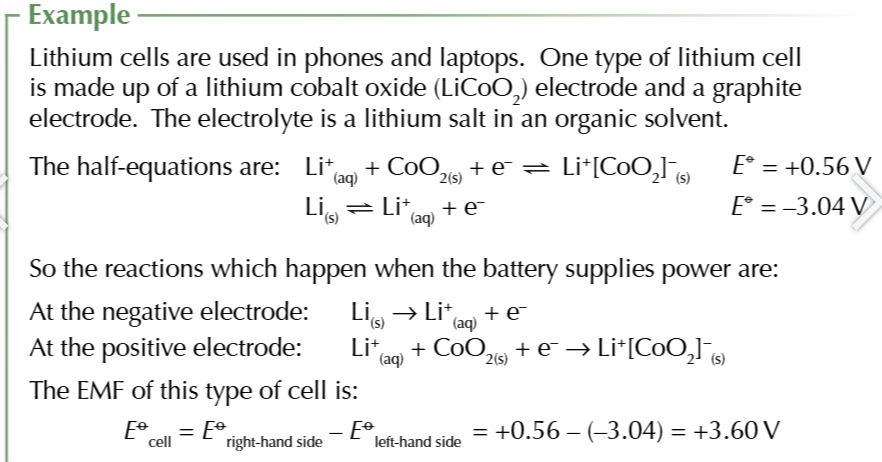
lithium cells
lithium cell batteries
Positive electrode: Lithium cobalt oxide (LiCoO2)
Negative electrode: graphite, where lithium ion reacts
electrolyte: lithium salt in organic solvent
half equations of lithium battery
negative electrode: Li → Li+ + e-
positive electrode: Li+ + CoO2 +e- → Li+[CoO2]-
there are 2 other types of rechargeable battery
NiCad (nickel-cadmium)
Lead-acid
for rechargeable batteries
a current is supplied to force electrons to flow in the opposite direction in the circuit which reverses the reaction
rechargeable reactions only work because
none of the substances in a rechargeable battery escape or get used up
the reactions that occur in non rechargeable batteries
are difficult or impossible to reverse in this way
for most cells
chemicals that generate the electricity are contained in the electrodes and electrolyte that form the cell
for fuel cells
chemicals are stored separately outside the cell and are fed in when electricity is required
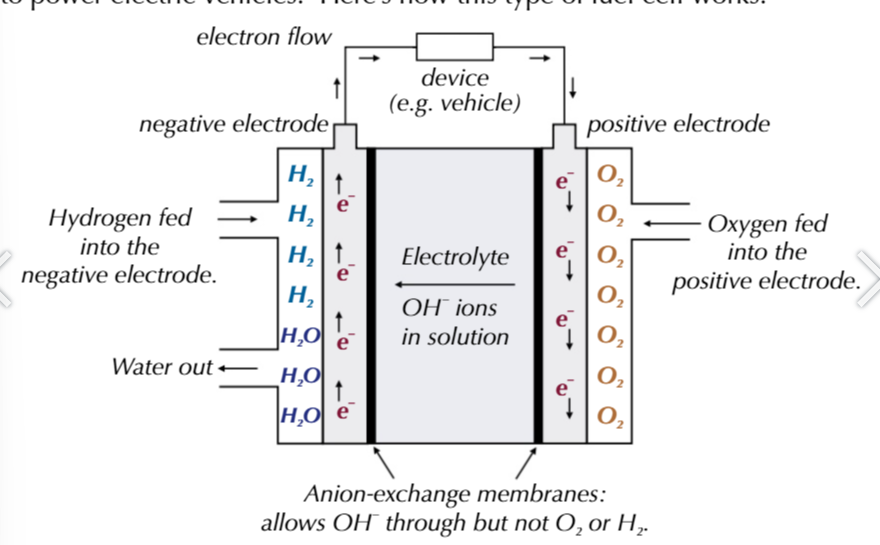
alkaline-hydrogen fuel cell powers electric vehicles, contain platinum electrodes
hydrogen and oxygen gases are fed into 2 seperate platinum-containing electrodes
these electrodes are made up by coating a porous ceramic material with a thin layer of platinum
this is cheaper than solid platinum rods and providesa larger surface area for a faster reaction
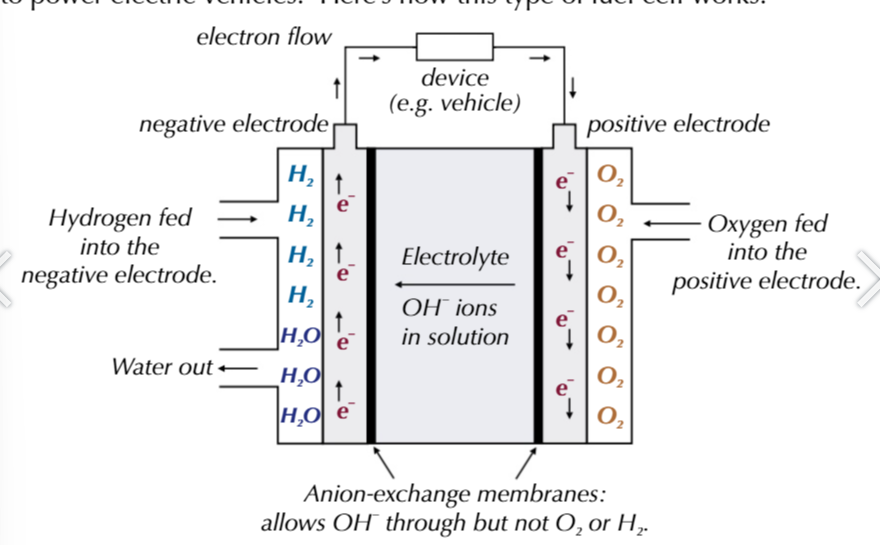
alkaline-hydrogen fuel cell, NEGATIVE ELECTRODE
the electrodes are separated by an anion-exchange membrane that allows anion (OH-) and water to pass through it
however it stopes hydrogen and oxygen gas from passing through it
the electrolyte is an aqueous alkaline KOH solution
hydrogen is fed to a negative electrode: H2 +2OH- → 2H2O + 2e-
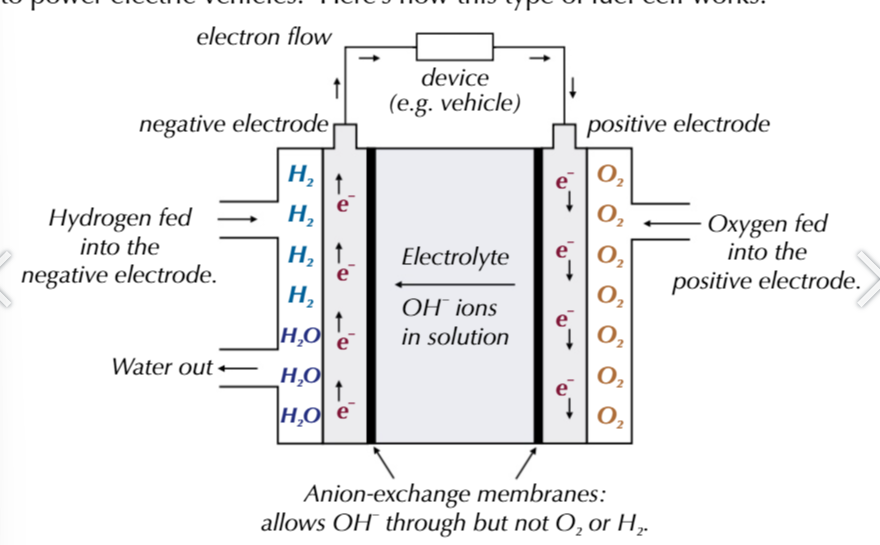
alkaline-hydrogen fuel cell
electrons flow from the negative electrode through an external circuit to the positve electrode
OH- pass through the anion-exchange membrane towards the negative electrode.
oxygen is fed to the positive electrode: O2 + 2H2O + 4e- → 4OH-
alkaline-hydrogen fuel cell overall reaction
2H2 + O2 → 2H2O
advantages of fuel cells in cars
fuel cells are more efficient, they convert more of their available energy into kinetic energy to get the car moving
internal combustion engines waste a lot of their energy producing heat
the only waste product of fuel cell is water, no toxic chemicals or CO2
fuel cells don’t need to be recharged like batteries
as long as hydrogen and oxygen are supplied the cell will continue to produce electricity
disadvantages of fuel cells
you need energy to produce a supply of hydrogen, produced from electrolysis of water but this requires electrocity thats normally generated from the burning of fossil fuels- this whole process is NOT carbon neutral
hydrogen is also highly flammable so needs to be handled carefully when stored and transported
infrastructure to prodive hydrogen fuel for cars doesnt exist on a large scale so refuling stations are very rare