Composition & yield
1/14
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Theoretical yield
maximum amount of product that can be made
Actual yield
The amount of product made by carrying out the reaction
Percentage yield equation
Actual yield/theoretical yield x 100
Reasons % yield is less than 100%
Reversible reaction, product is lost, side reactions
Reversible reaction
A chemical reaction in which the products re-form the original reactants
Ways product is lost
Stuck in the reaction vessel, or by separation from the reaction mixture
Side reactions
Reactions that do not give the desired product of a reaction
Use of percentage yield
To measure the efficiency of a reaction
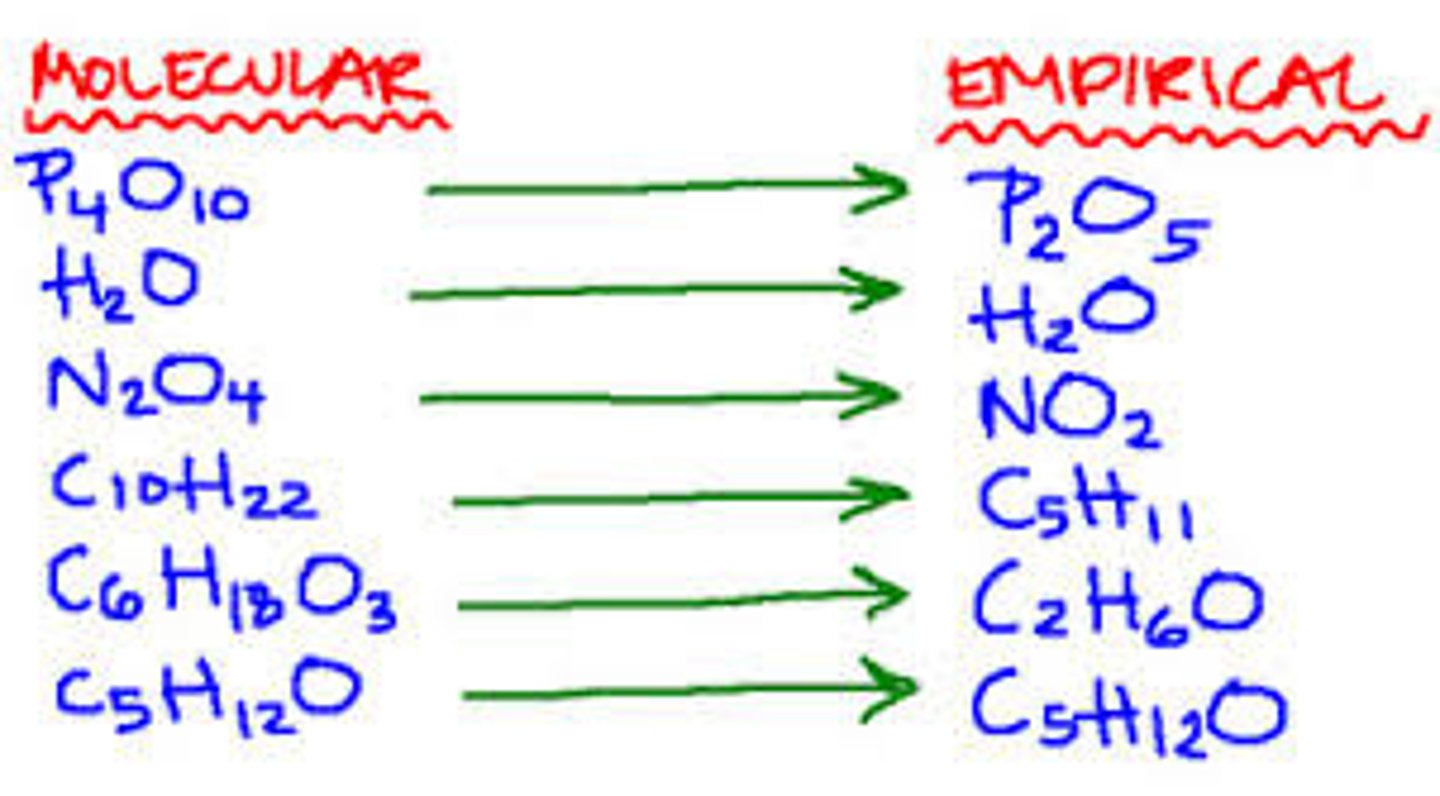
Molecular formula
A chemical formula that shows the number and kinds of atoms in a molecule, but not the arrangement
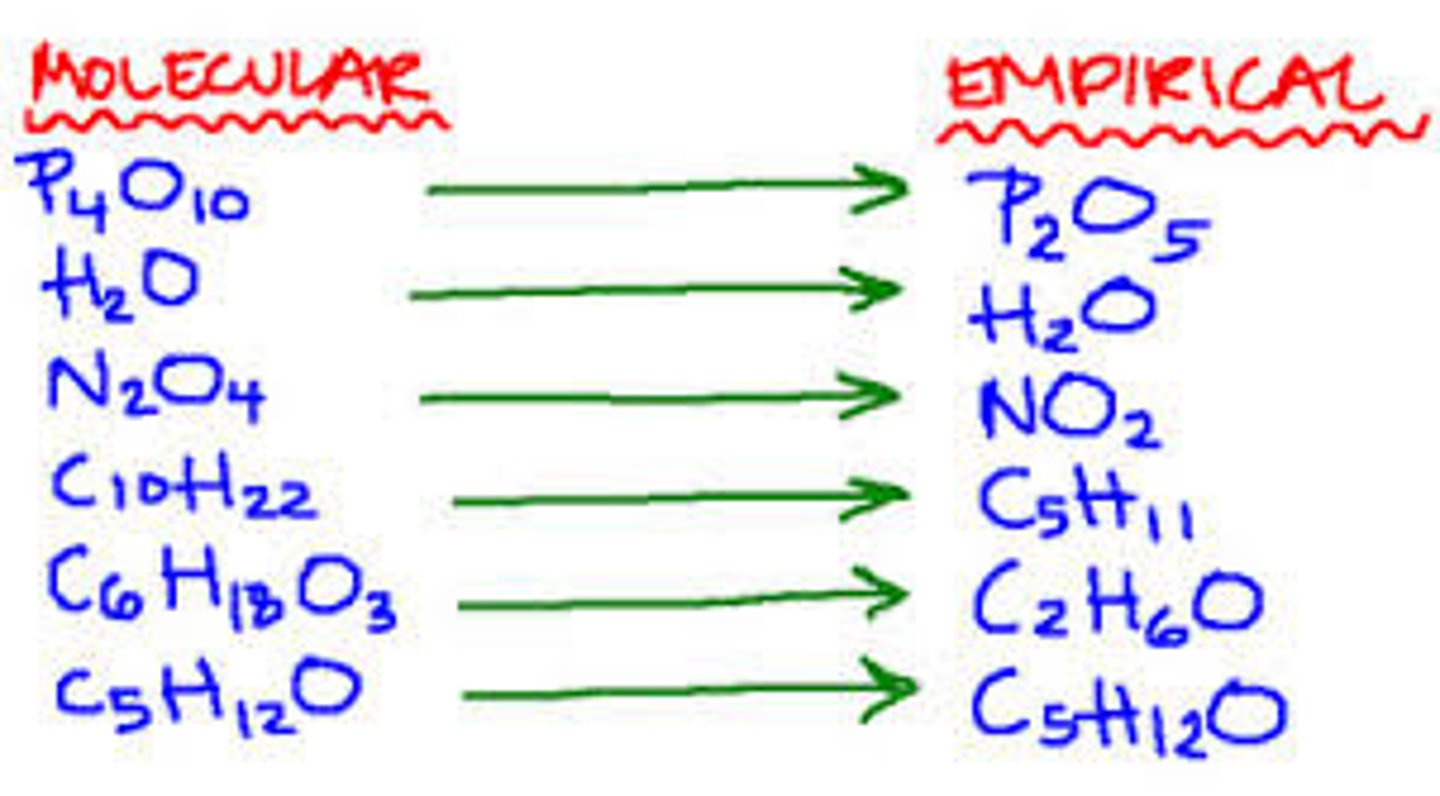
Empirical formula
a chemical formula showing simplest ratio of elements in a compound
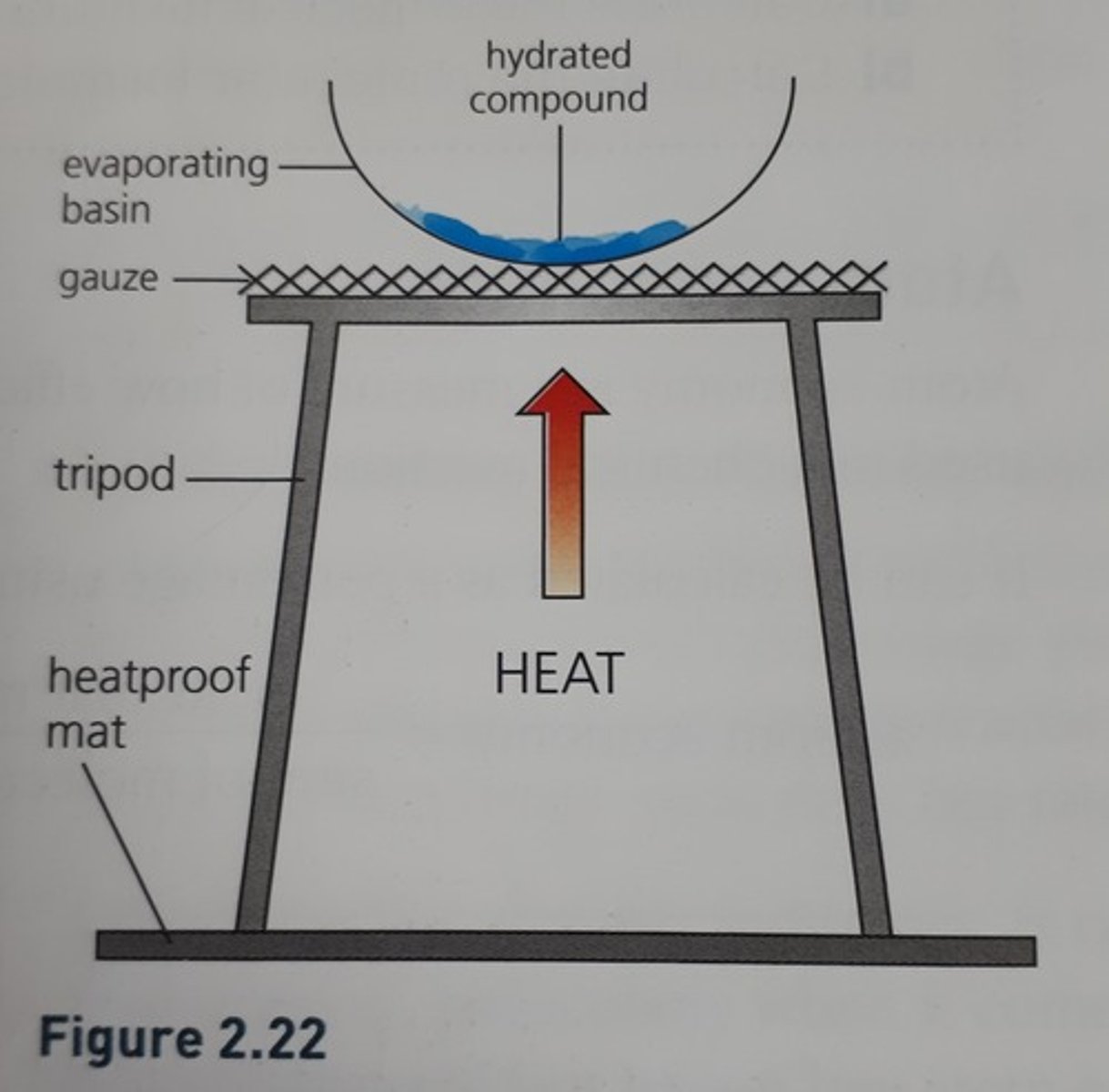
Hydrated
A crystal containing water molecules

Anhydrous
A crystal which doesn't contain any water molecules

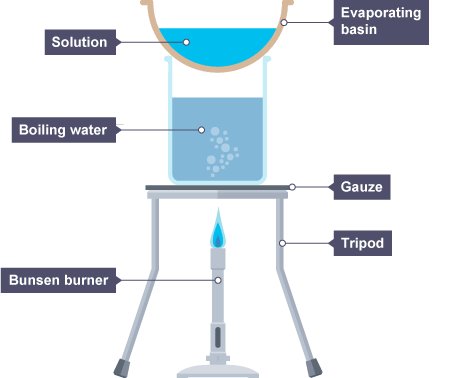
Heating to constant mass
Heating and weighing, and repeating this until two readings are the same
Thermal decomposition
The breakdown of a compound by heat
Determining waters of crystallisation
Heat hydrated compound to constant mass