6. Colloidal instability, flocculation and rheology of dispersions
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Instability in colloidal dispersions
Sedimentation
Creaming
Flocculation (aggregation)
Ostwald ripening
What is sedimentation and creaming?
Sedimentation: Particle density is higher than the density of continuous phase → particles sink
Creaming: Particle density is lower than the density of continuous phase → particles rise to surface
Flocculation/aggregation
a process where a solute (particles) comes out of solution in the form of floccules due to insufficient repulsion between particles
Ostwald ripening
Large particles grow at the expense of smaller ones. Depends on how soluble dispersed phase is in continuous phase, size and laplace pressure
Smaller droplets → higher laplace pressure → higher driving force
At which concentration is Stokes law a good estimation of real sedimentation?
Low concentration (φ<5%): vsed = vStokes
For dispersion by particles is 25%
High concentration: (φ>5%): vsed< vStokes
Sedimentation rates are lower than Stoke’s law predict
Hindered sedimentation/creaming
The sedimentation or creaming rate decreases with increasing concentration of the dispersed phase.
When we have more 40% sedimentation doesn’t occur since there is no space, the particles are too crowded
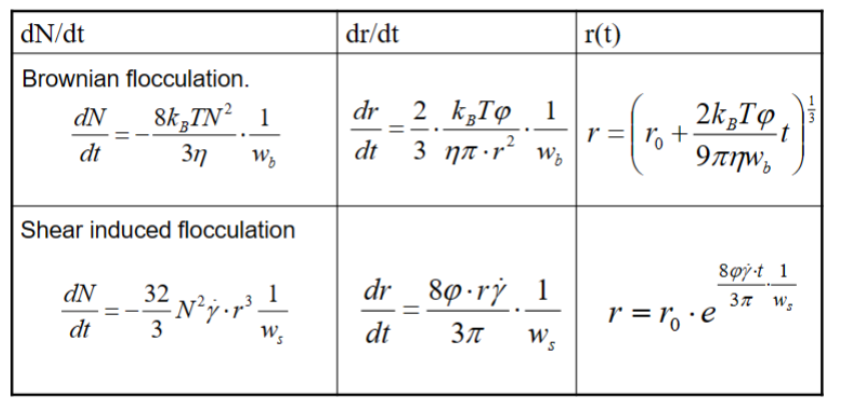
There are three mechanisms for flocculation:
• Brownian motions
The particles get close to each other through random movement
• Shear induced flocculation
Some particles experience higher or lower shear stress depending on their surface
• Gravitity induced flocculation
Comparison between Brownian and shear flocculation rate
Brownian:
Decreases with increased size
Decreases with increased viscosity
Shear:
Increases with increased size
Increases with increased shear rate
N = number concentration of particles, kB = Boltzmann constant, T = absolute temp., η =
dynamic viscosity, w = stability factor, φ = volume fraction of particles, γ = shear rate
Stability factor w
w=1 → each collision leads to aggregation (high possibility of flocculation)
w=106 → one collision in a million leads to aggregation
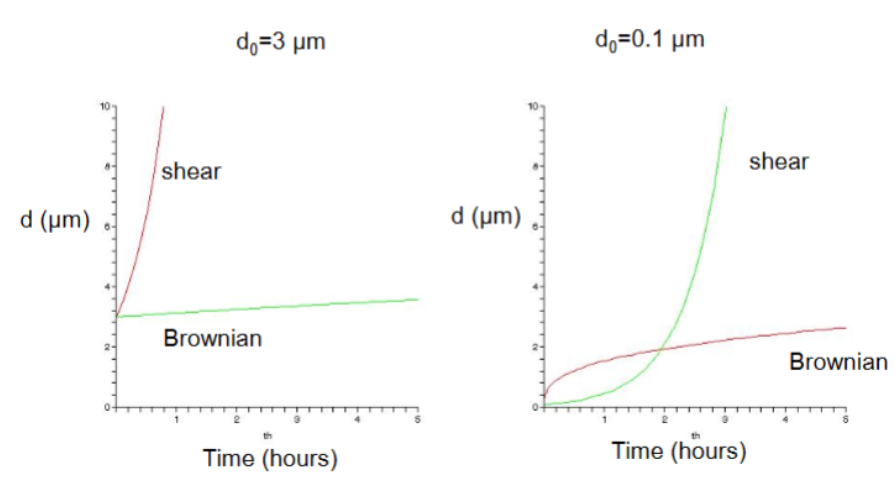
Comparison between shear and brownian flocculation
do=0.1 μm
Small particles flocculate quickly due to Browian but then it is too big and slow down
Brownian: Initially rapid, but then slowly
Shear: In the beginning, it is slow, but then rapidly increases
do=3 μm
Shear: Rapid growth of particle size. Large and likely to meet than small particles in shear-like movement.
Browian: Slow. Larger particles have a slower diffusion so it takes them longer to randomly meet
Fractal aggregates (flocs)
Aggregates that occupy a larger volume than the individual particles are called fractal aggregates or flocs
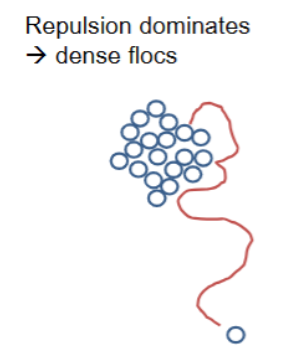
Repulsive interactions and aggregate structure
Repulsion dominates → dense flocs
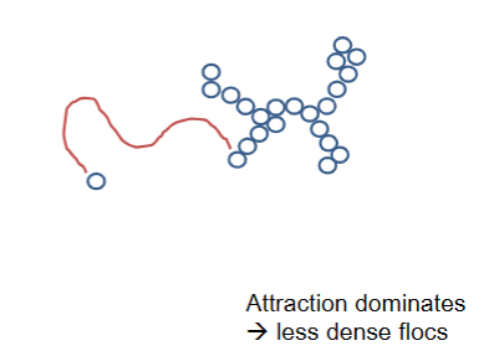
Attractive interactions and aggregate structure
Attraction dominates → less dense flocs
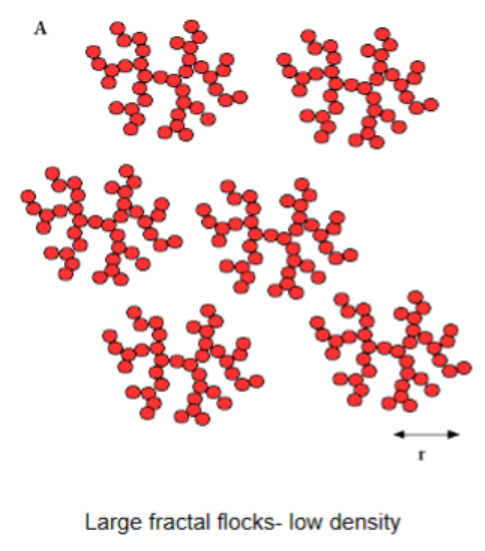
Larger fractal flocks
Larger structure but lower density → more rapid sedimentation
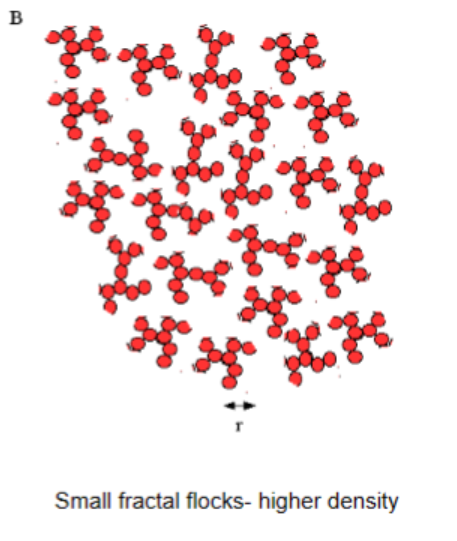
Smaller fractal flocks
Higher density
Ostwald ripening and laplace pressure
Ostwald ripening riven by the Laplace pressure
• Molecules from smaller particles dissolve and diffuse to larger particles
• Occurrence and rate also depend on the solubility disperse phase molecules in the continuous phase
– Low solubility slows it down or inhibits it
• Also referred to as ”disproportionation” in foams
We have pressure and high surface tension. We need solubility
If this has low solubility in the continuous phase, process slowed down
Dispersion rheology
Higher viscosity is the result of particles disturbing the applied flow field and particles ”bumping into each other”.
In pure liquids: viscosity is the result of friction between molecules.
Relative viscosity relation to volume fraction
Higher volume fraction —> higher relative viscosity
How does aggregation/flocculation affect viscosity?
Dispersed particles in continuous phase
Flocculation occurs → Viscosity decreases
How does breaking up aggregates affect viscosity?
More particles flowing around bumping into each other → Viscosity increases