Chemistry ch4
0.0(0)
Card Sorting
1/37
Last updated 12:55 AM on 10/13/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
38 Terms
1
New cards
How is a solid different from a liquid ?
The solids particles are rigid, they don't flow
2
New cards
Phase change from solid to liquid
melting
3
New cards
phase change from liquid to solid
freezing
4
New cards
phase change from solid to gas
sublimation
5
New cards
phase change from gas to solid
deposition/desublimation
6
New cards
how are the properties of solids like liquids'?
they reflect the attraction and arrangement of their particles
7
New cards
what is the melting/freezing point for ionic solids?
HIGH
8
New cards
ionic solids
opposite charges (positive+negative) {alex+layla}
9
New cards
what is the attraction strength for ionic solids?
STRONG
10
New cards
what is the attraction strength for molecular solids?
WEAK
11
New cards
Melting point for molecular solids
LOW
12
New cards
what elements are involved with ionic solids?
metals+nonmetals
13
New cards
what elements are involved with molecular solids?
nonmetals+nonmetals
14
New cards
most solids form a 3D pattern called what?
a crystal lattice
15
New cards
Unit cell
the smallest group of particles within a crystal that retains the geometric shape of the crystal
16
New cards
amorphous solids(glass, rubber)
lacking form
17
New cards
Allotropes(carbon, phosphorus)
two or more molecular forms in the same element and physical state
18
New cards
Which compound has the higher melting point
silver chloride (AgCl) or water (H20)?
silver chloride (AgCl) or water (H20)?
silver chloride, because it is an ionic solid the melting point is higher.
19
New cards
which compound has the higher melting point
silicone dioxide (SiO2) or Iron chloride (FeCl)?
silicone dioxide (SiO2) or Iron chloride (FeCl)?
Iron chloride, because it is a ionic solid so the melting point is higher
20
New cards
what phases are in equilibrium at a substances melting point?
solid and liquid
21
New cards
List the states of matter correctly in order of increasing average kinetic energy
Solid, liquid, gas.
22
New cards
mol(e)
a set of number of particles in a substance
23
New cards
what is the gas law that has no constants, but combines all three variables?
combined gas law
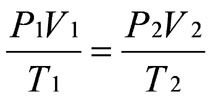
24
New cards
what gas law has gas constant, and number of moles?
ideal gas law
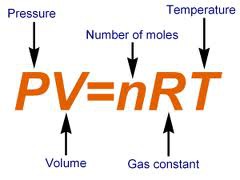
25
New cards
what gas law has volume constant, and is proportional?
gay-lussacs
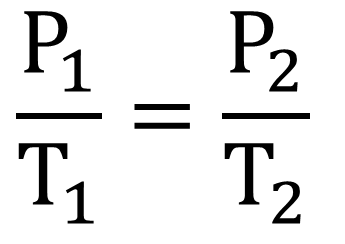
26
New cards
what gas law has temperature constant, and is inversely proportional?
boyle's law
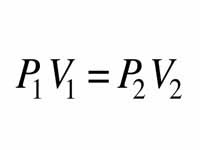
27
New cards
what is the gas law that has pressure constant and is proportional ?
Charles' law
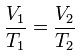
28
New cards
What are the two major assumptions that are needed for a gas to be ideal? There what can be assumes about all gases?
1. particles take up no volume
2. particles don't interact
no gases are ideal
2. particles don't interact
no gases are ideal
29
New cards
how is a liquid different than a gas?
liquid particles are connected together, and are not easily compressible
30
New cards
A liquid is characterized by
the attractions that hold it together
31
New cards
volatility
describes how easily a substance will vaporize
32
New cards
What is room/normal pressure?
1 atm
33
New cards
barometer
measure the atmosphere pressure
34
New cards
manometer
measures differences between pressure
35
New cards
why is pressure lower at higher altitudes?
Gravity prevents less air particles to go to higher altitudes, so there is lower pressure
36
New cards
what is pressure?
force per area
37
New cards
what is temperature?
amount of kinetic energy of a particle
38
New cards
what is volume?
amounts of space something takes up