cell- signalling RTK
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
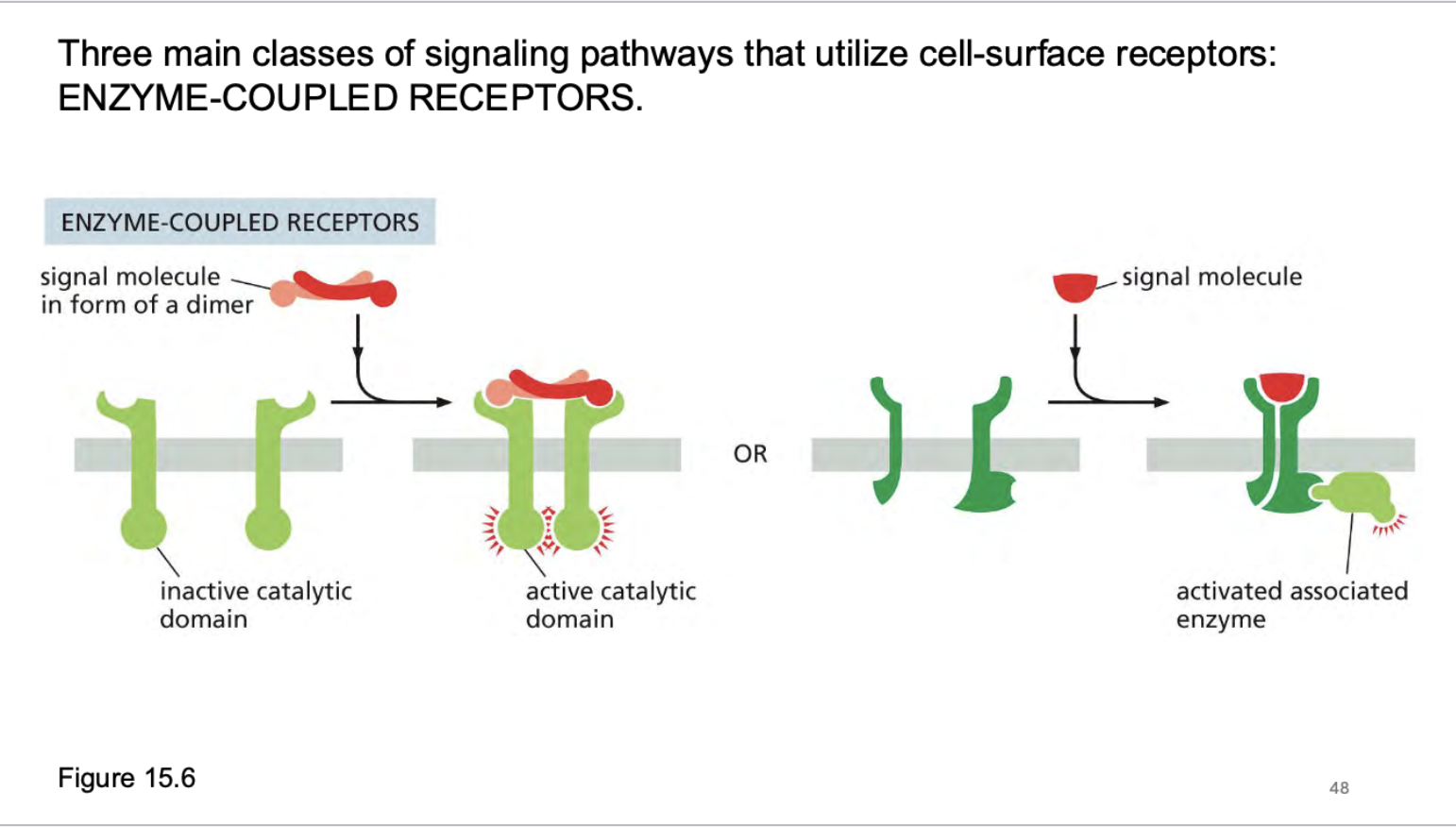
how does a enzyme coupled receptor work?
what family of proteins is a target for several anti cancer drugs?
RTK family
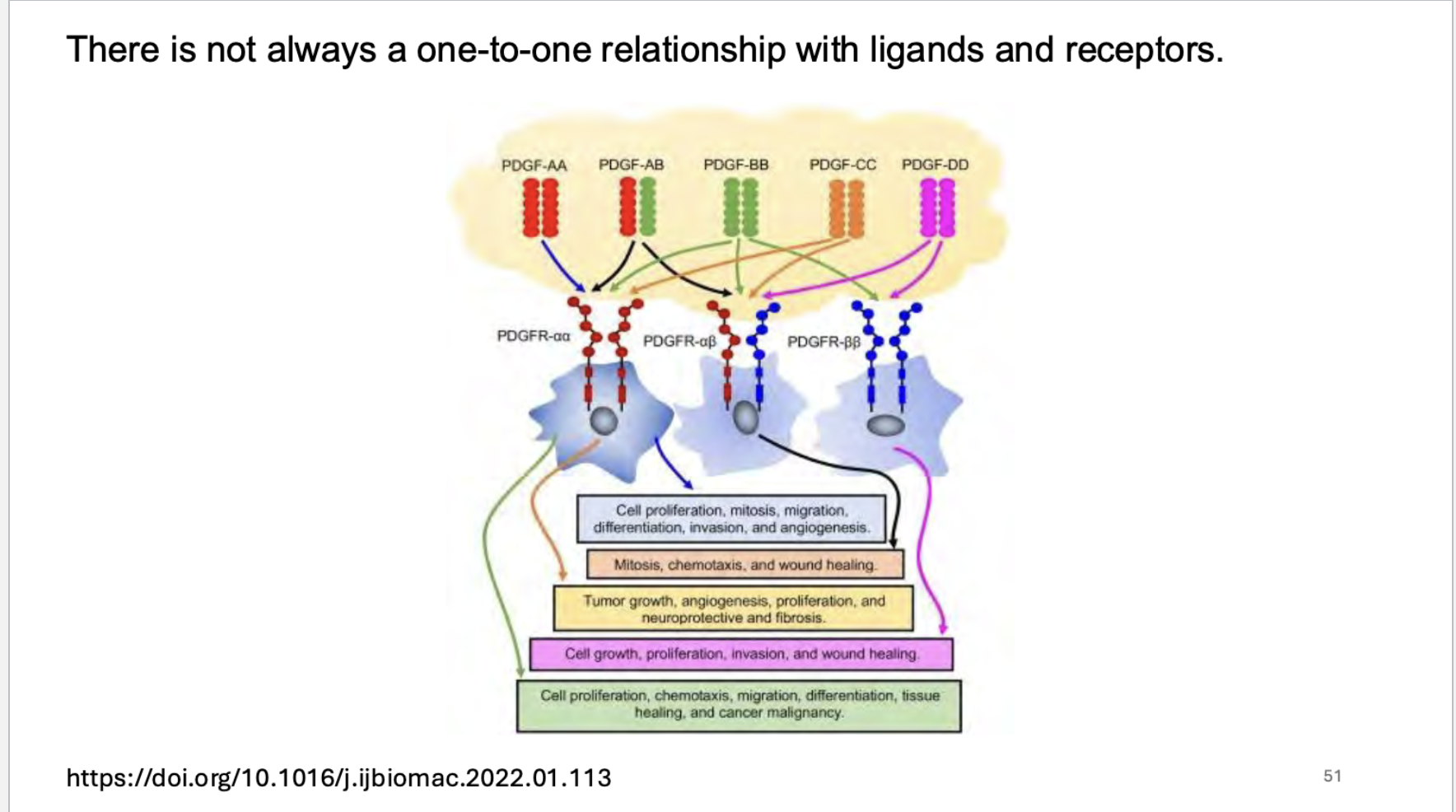
is there a one to one relationship with ligands and receptors
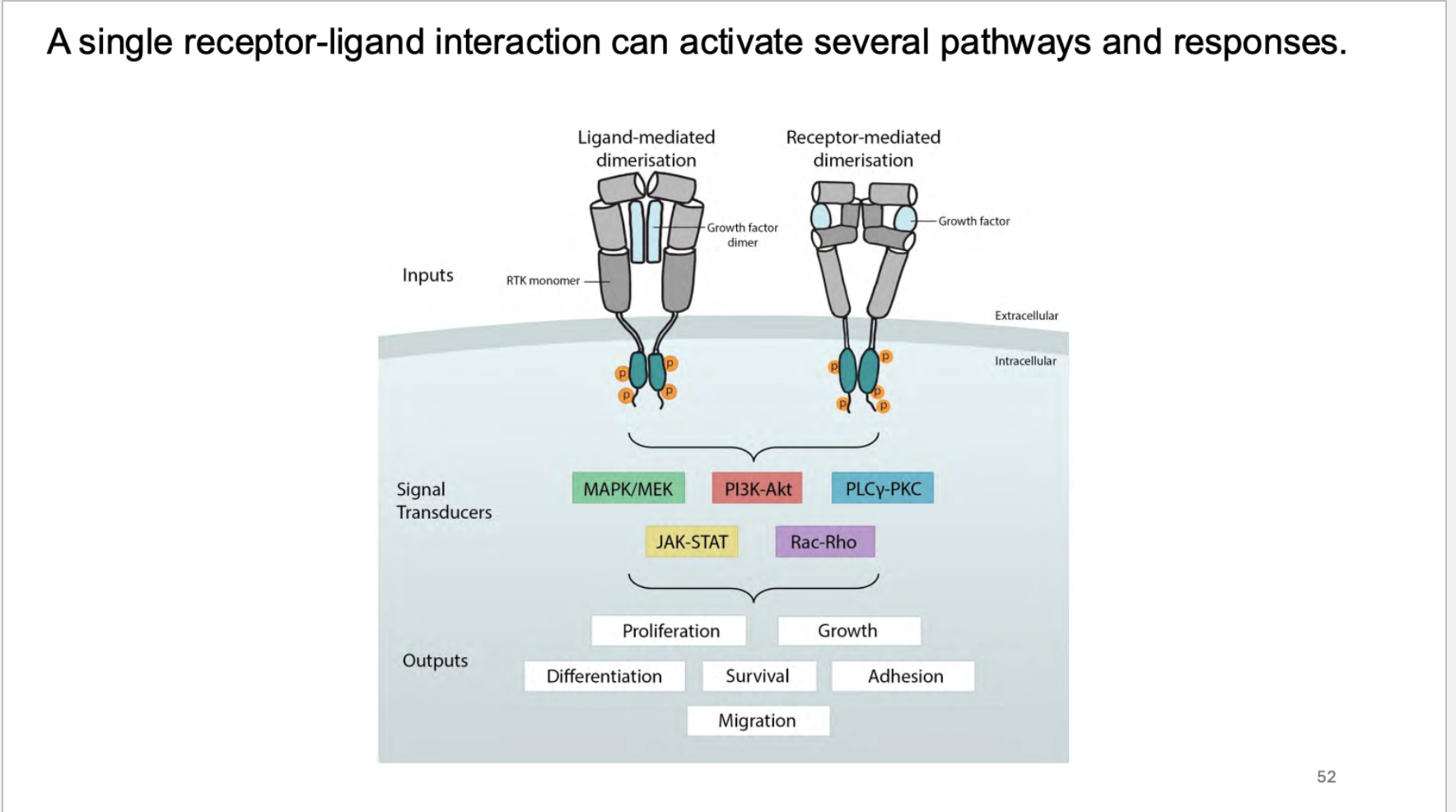
how many pathways can a single receptor ligand interaction activate?
What are receptor tyrosine kinases regualated by? What are the steps to their activation?
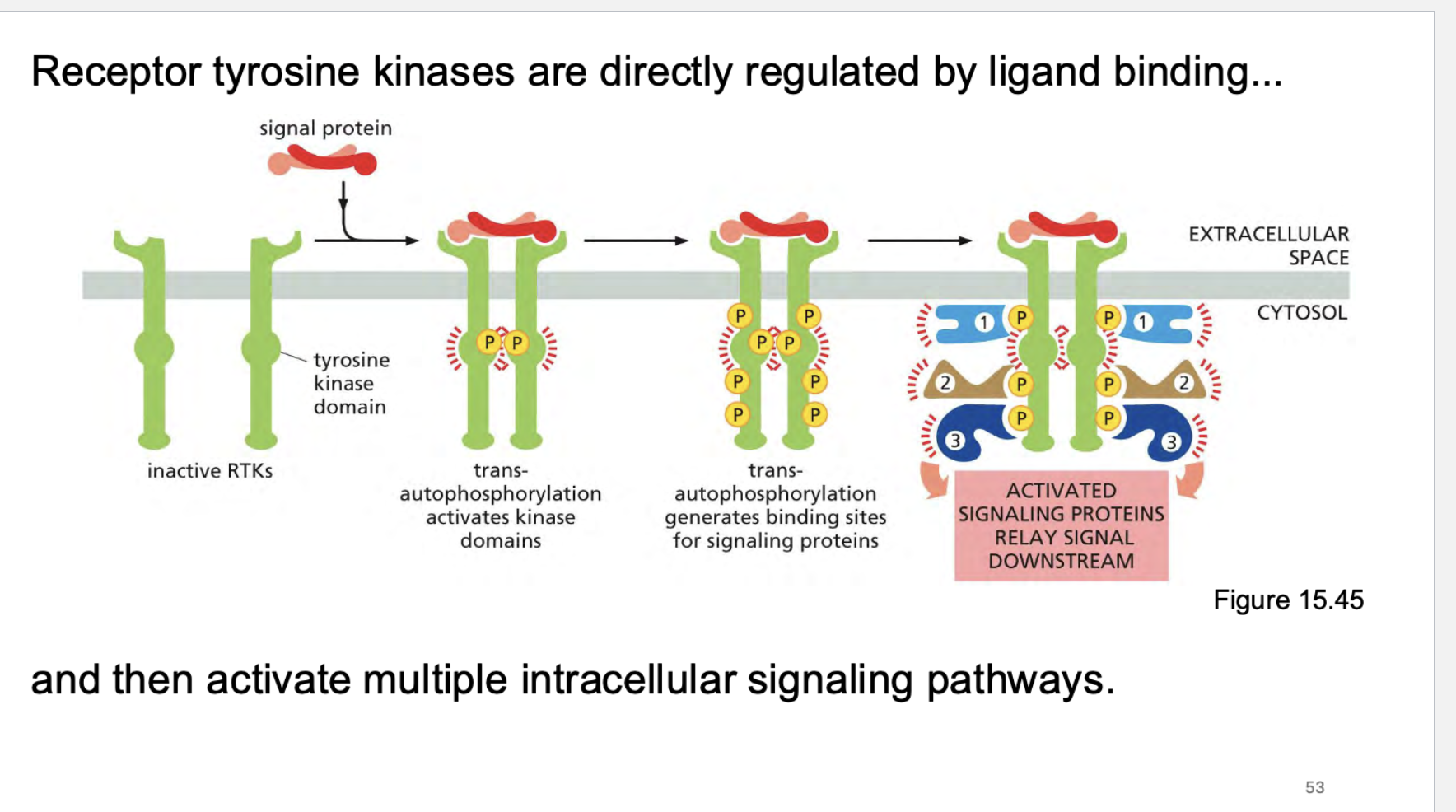
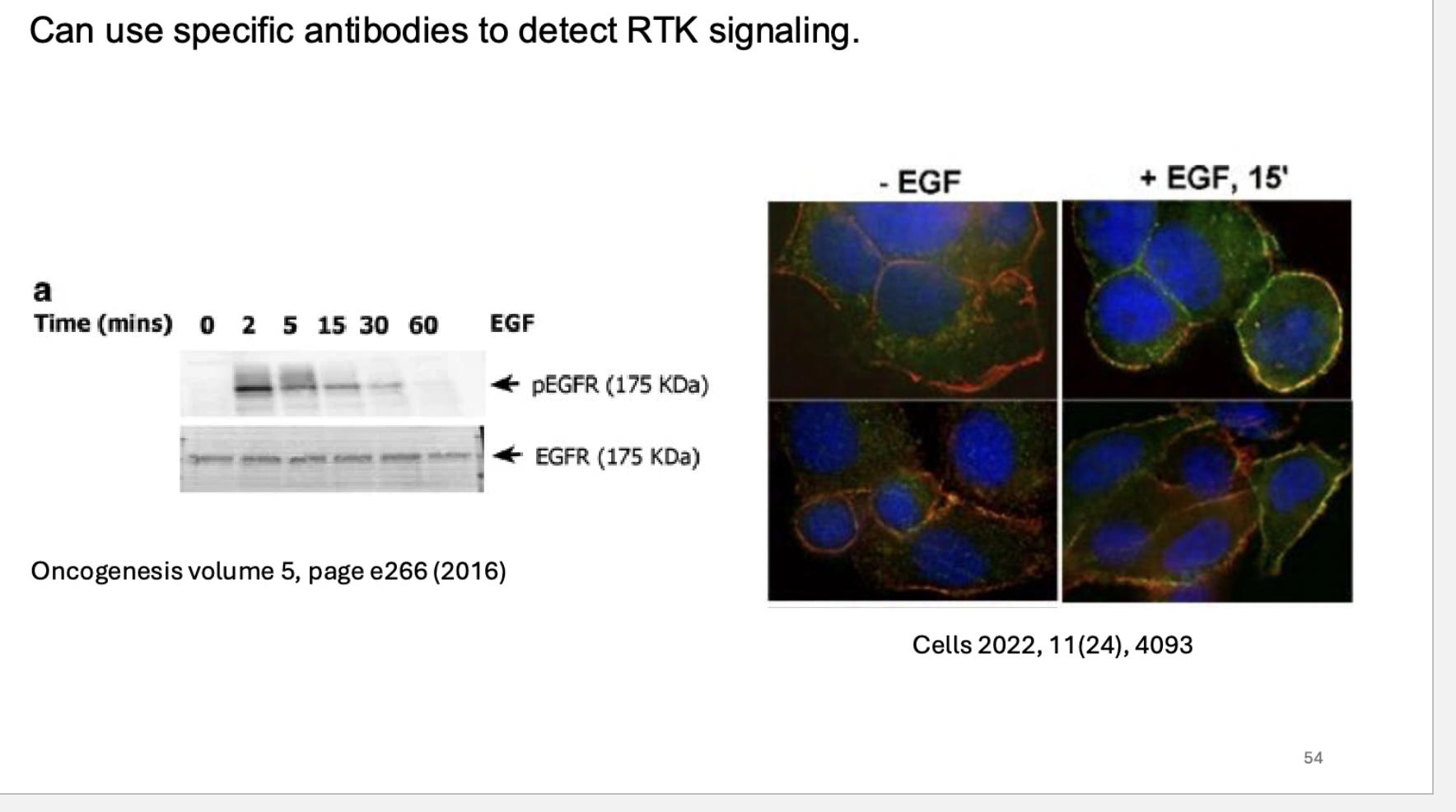
what can we do to detect RTK signaling?
use specific antibodies.
how do rtk work? what do they activate? What are the domains that bind different things?
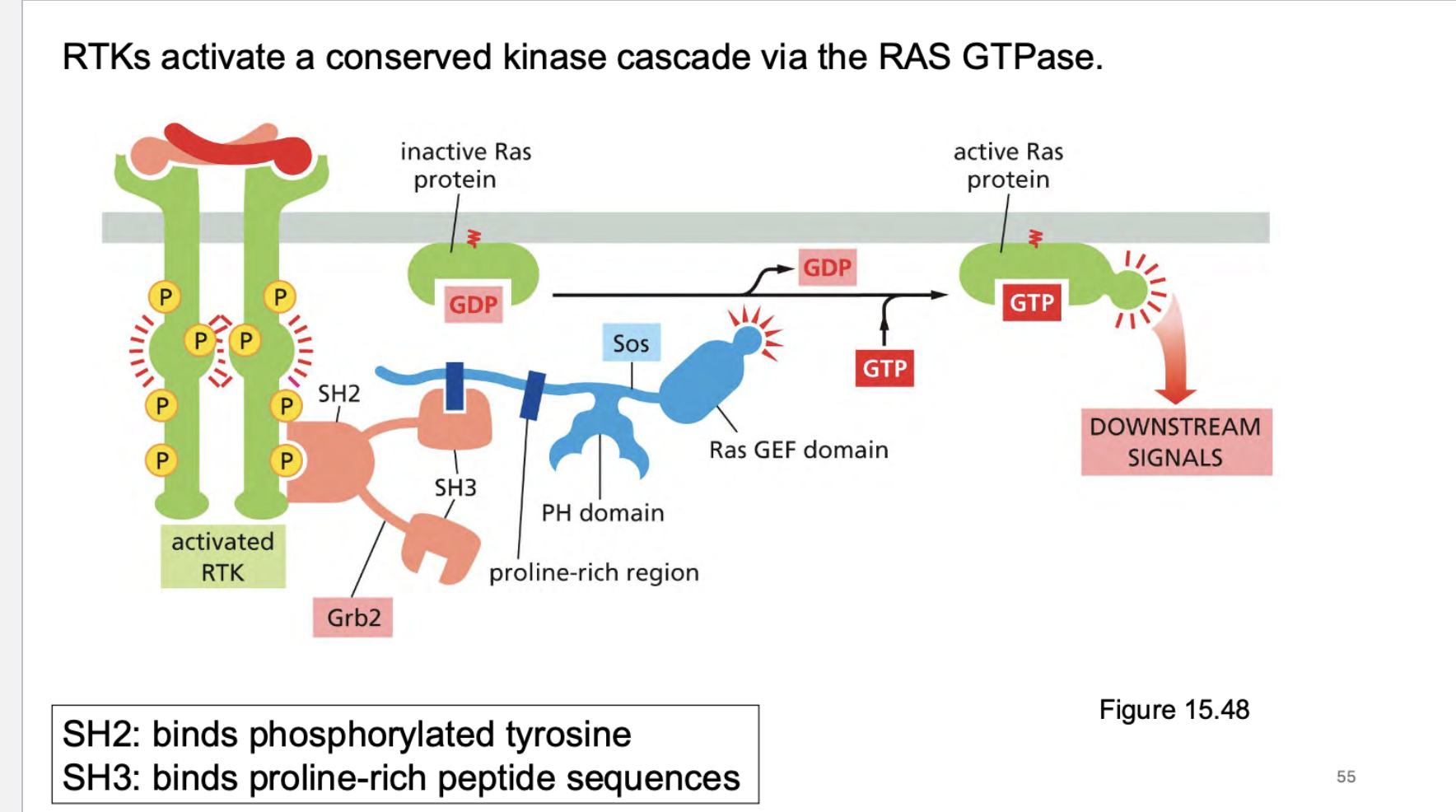
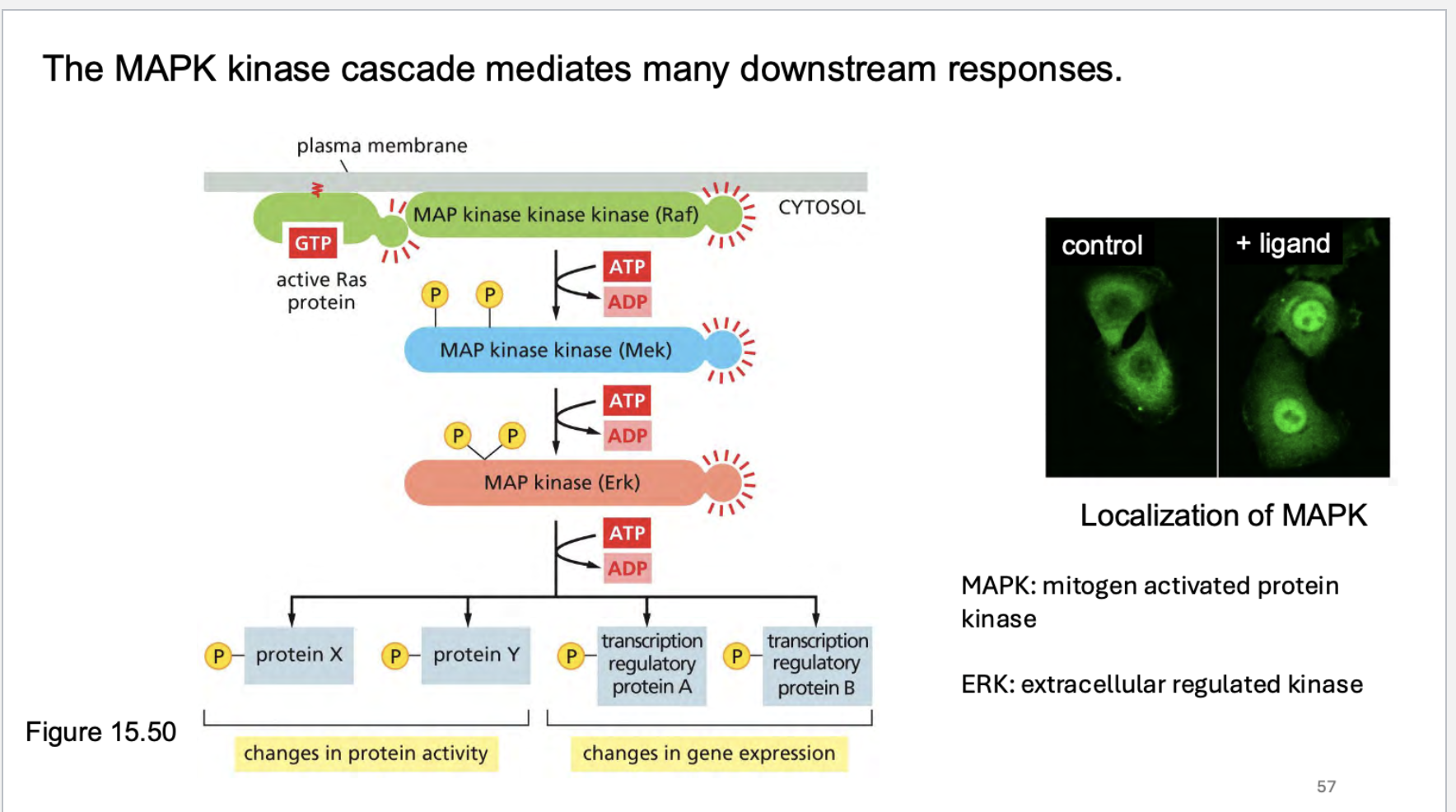
Growth factor ➔ RTK ➔ Grb2/SOS ➔ RAS-GTP ➔ RAF ➔ MEK ➔ ERK ➔ Gene expression
what do conserved domains do?
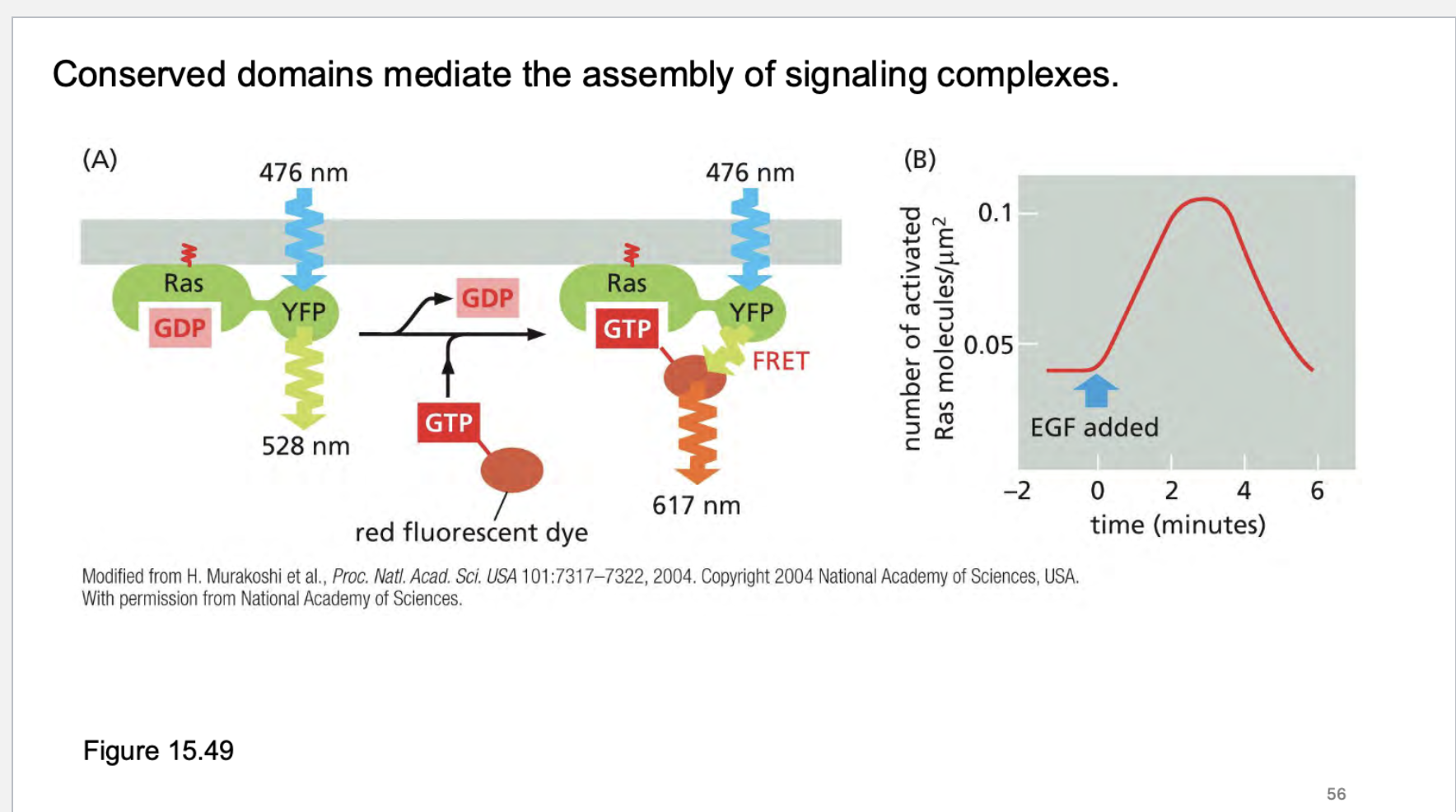
RAS GAP = RAS GTPase-Activating Protein.
It’s the "brake" on RAS signaling.
RAS on its own is a slow GTPase — it eventually hydrolyzes GTP to GDP and turns itself off, but it’s slow.
RAS GAPs speed up this hydrolysis:
They bind to active RAS-GTP using a RAS-binding domain.
They help RAS hydrolyze GTP to GDP much faster.
This turns RAS back to its OFF state (RAS-GDP).
In short:
RAS GAPs terminate RAS signaling by accelerating GTP hydrolysis, turning RAS off.
go through the map kinase cascade and what it does? What is MAPK stand for? What does ERK stand for? What happens
rtk signaling is dependent on what?

EGF binds EGFR ➔
EGFR dimerizes and autophosphorylates (p-EGFR) ➔
p-EGFR recruits adapter proteins (like Grb2, SOS) ➔
This activates RAS (by exchanging GDP for GTP) ➔
RAS then activates further downstream pathways (like RAF → MEK → ERK).
How much EGF is present matters — higher EGF concentrations can cause more EGFR activation, stronger and longer RAS signaling.
How long the EGFR remains active (phosphorylated) and how long RAS stays active matters too — short pulses versus long sustained signaling can lead to very different cellular outcomes (for example, transient vs. sustained ERK activation leads to cell proliferation vs. differentiation).
what are phosphoinositides? What purpose do they serve? What are they catalyzed by? What are they bound by?
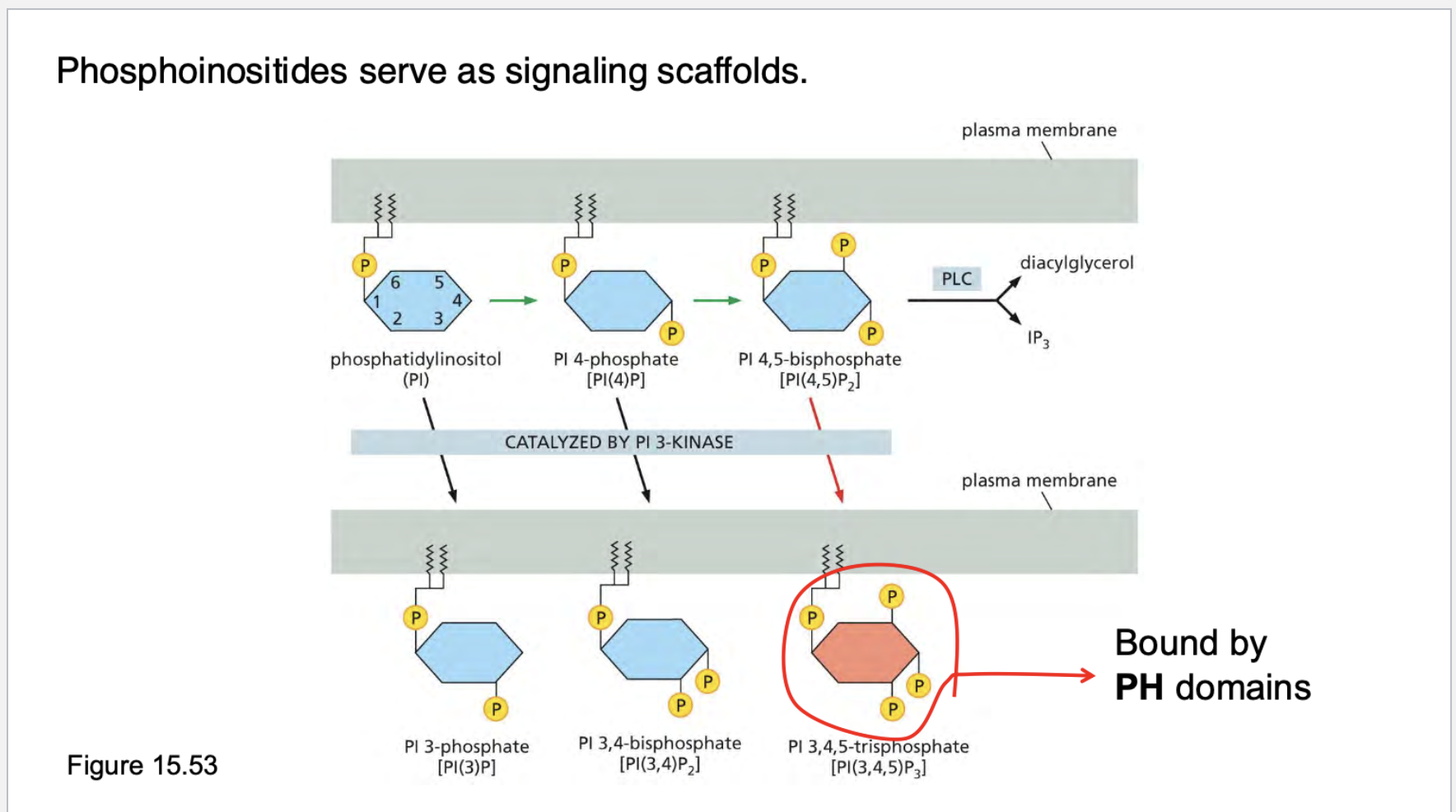
. Signaling platforms ("zip codes" on membranes):
Phosphoinositides recruit signaling proteins to specific locations.
Example: PH domains on proteins (like AKT) specifically bind PIP₃ (PI(3,4,5)P₃).
🔹 2. Control membrane identity and trafficking:
Different organelles have different "signatures" of phosphoinositides.
This helps control endocytosis, exocytosis, membrane remodeling.
🔹 3. Trigger downstream signals:
PIP2 can be cleaved by PLC into:
IP3 (increases intracellular calcium)
DAG (activates PKC)
🔹 4. Regulate cytoskeleton dynamics:
PIP2, PIP3 control how actin fibers organize — affects cell shape, migration.
how do phospholipids work? What do they do? Go through the pathway. What is PTEN
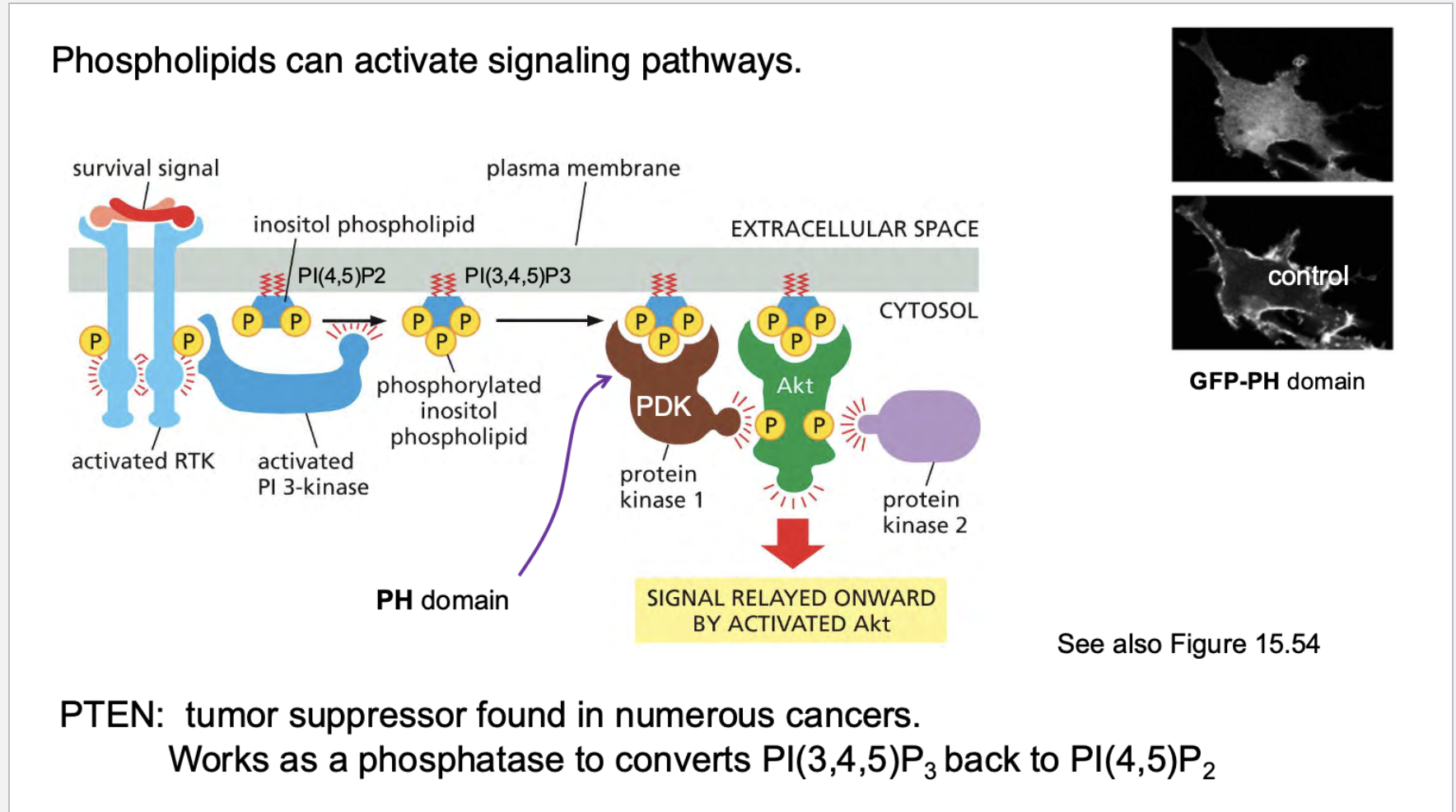
Step | Description |
|---|---|
1 | RTK gets phosphorylated (activated) |
2 | PI3K binds via SH2 domain |
3 | PI3K converts PIP2 ➔ PIP3 |
4 | PIP3 recruits PDK1 and AKT |
5 | AKT gets activated |
6 | AKT promotes survival, growth, metabolism |
The tumor suppressor PTEN is a phosphatase that removes the phosphate from PIP3, turning it back to PIP2 — basically shutting off this signaling.
If PTEN is lost (which happens in many cancers), the PI3K-AKT pathway stays on all the time, making cells immortal.
what does AKT regulate?
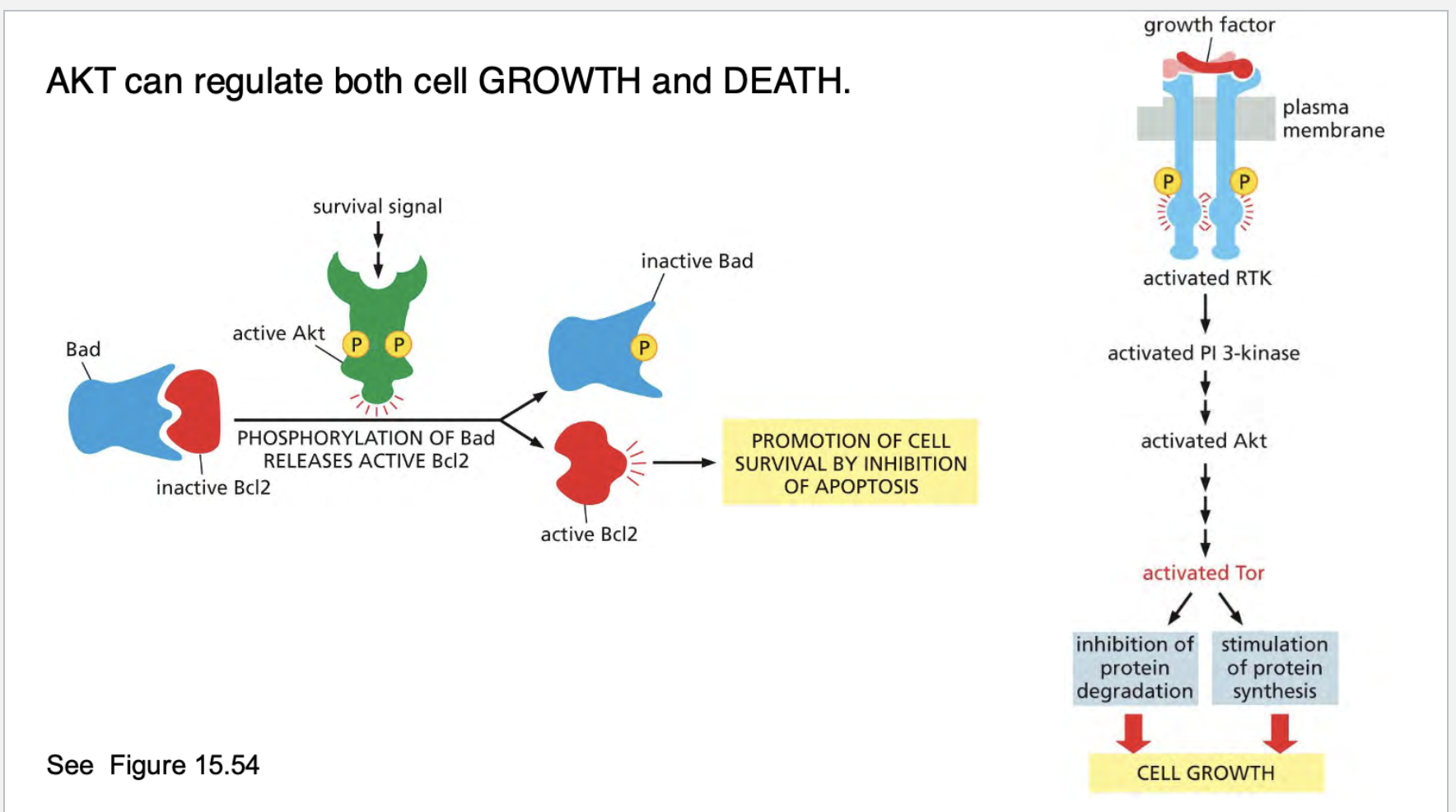
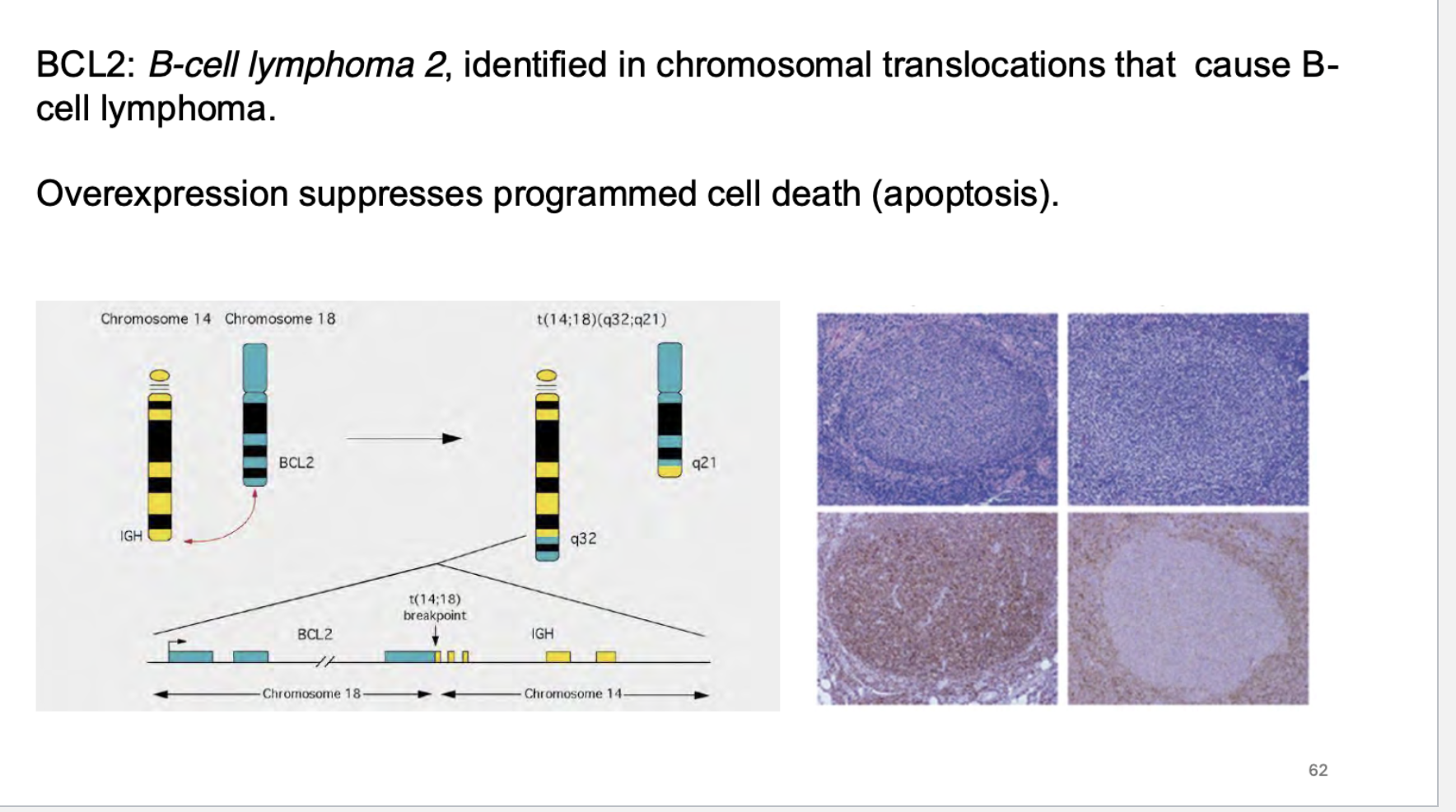
what does BCL2 do? How was it identified? What does over expression of this gene do?
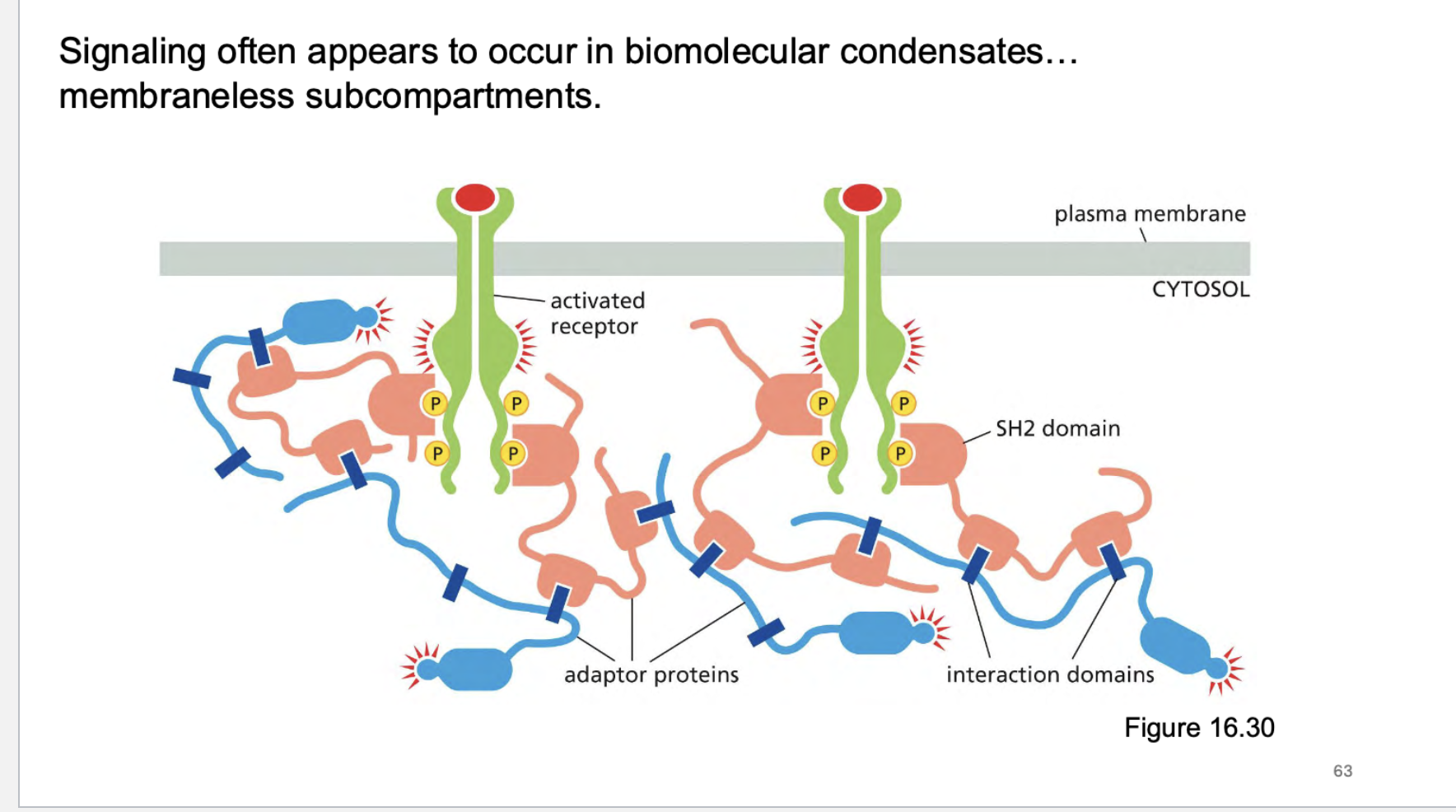
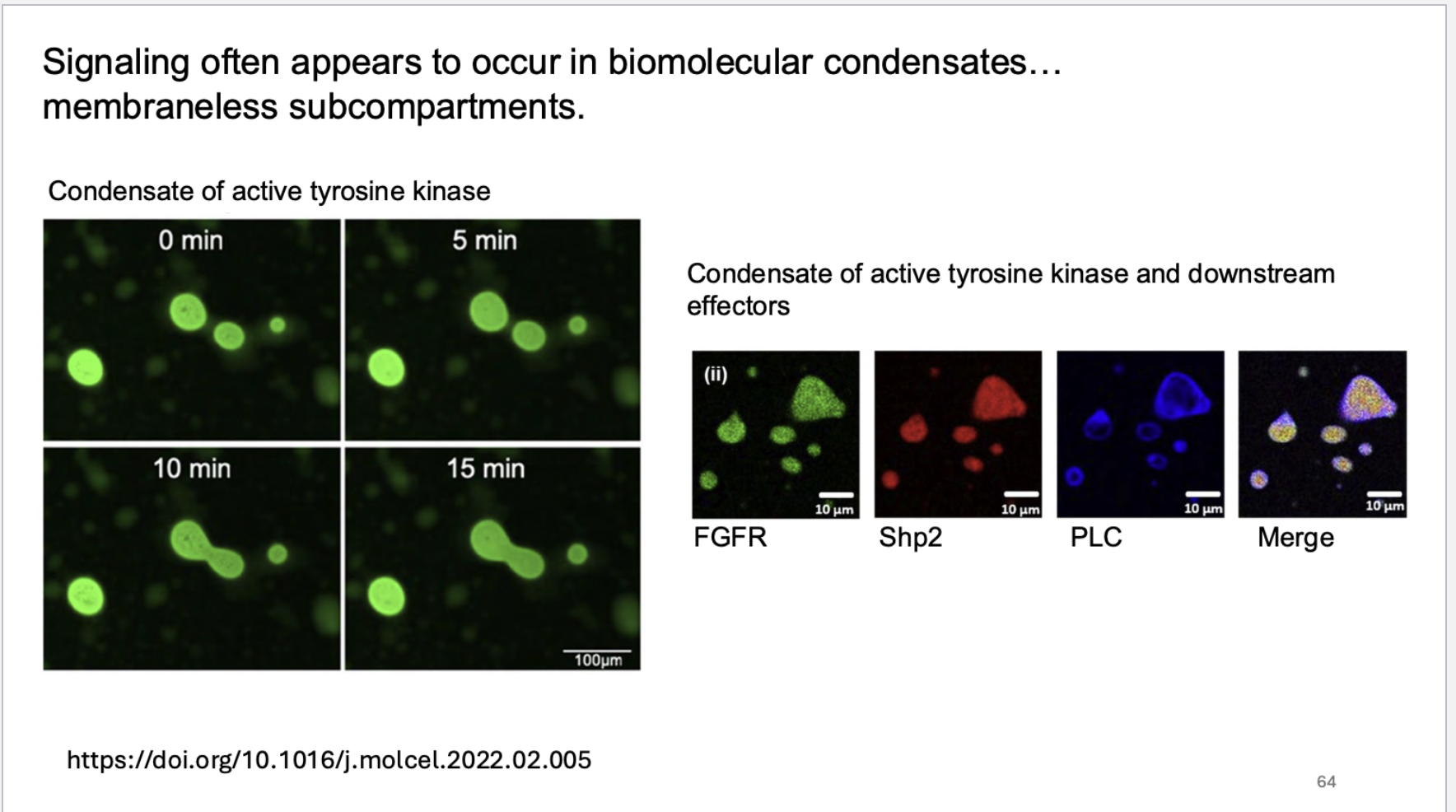
where does signaling often appear to occur? How does this work?
An SH2 domain (Src Homology 2 domain) is a specialized protein domain that recognizes and binds to phosphorylated tyrosine residues (p-Tyr) on other proteins.
Here’s the idea in simple terms:
When a receptor like EGFR gets activated, it becomes phosphorylated on tyrosines (meaning phosphate groups are stuck onto specific tyrosine amino acids).
Proteins that contain SH2 domains can dock onto these phosphorylated tyrosines.
This docking is very selective — SH2 domains don't just bind any phosphorylated tyrosine; they also recognize a few amino acids next to the p-Tyr to make it specific.
review the RTK signaling pathway

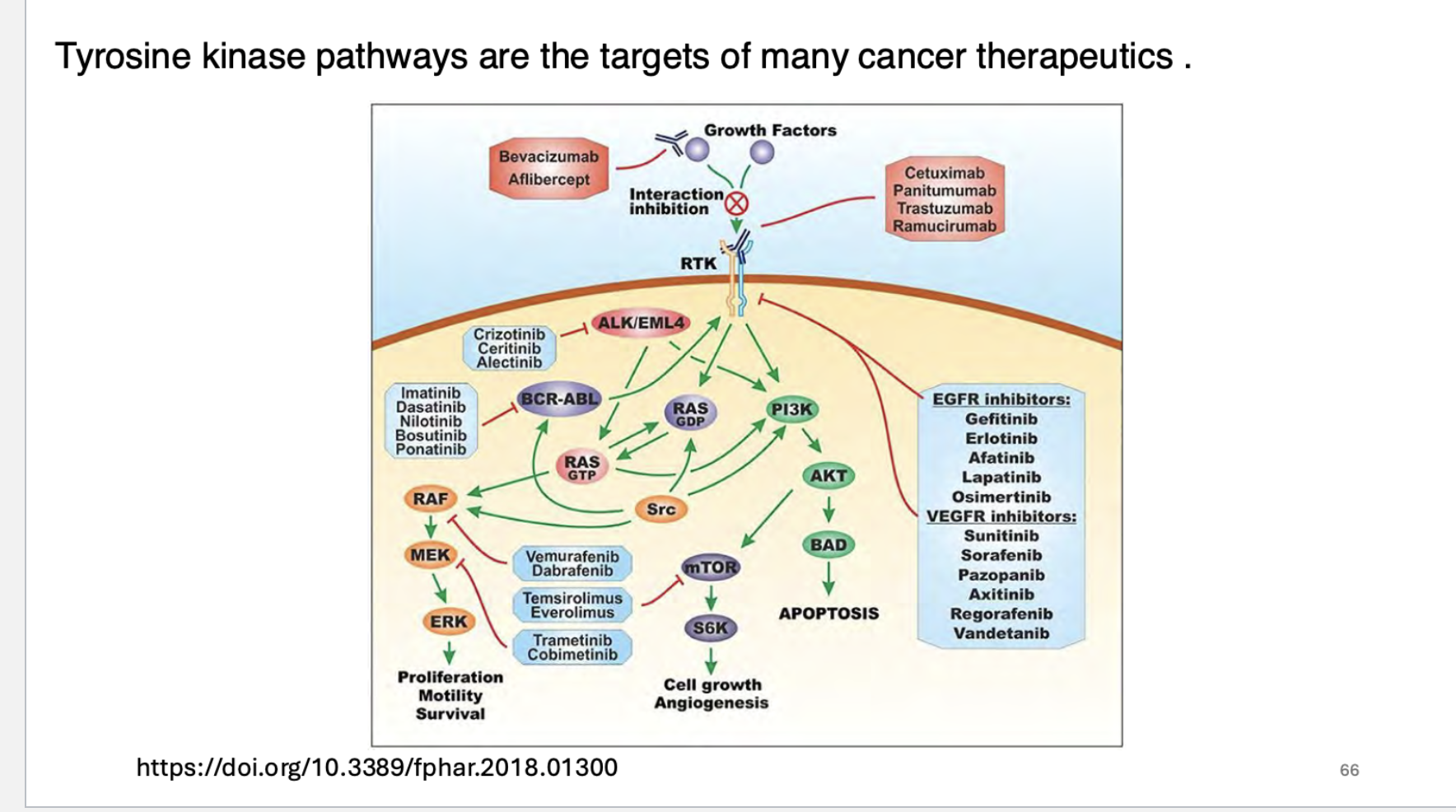
what pathways are the targets of many cancer therapeutics?
what happens to cancer cells eventually?
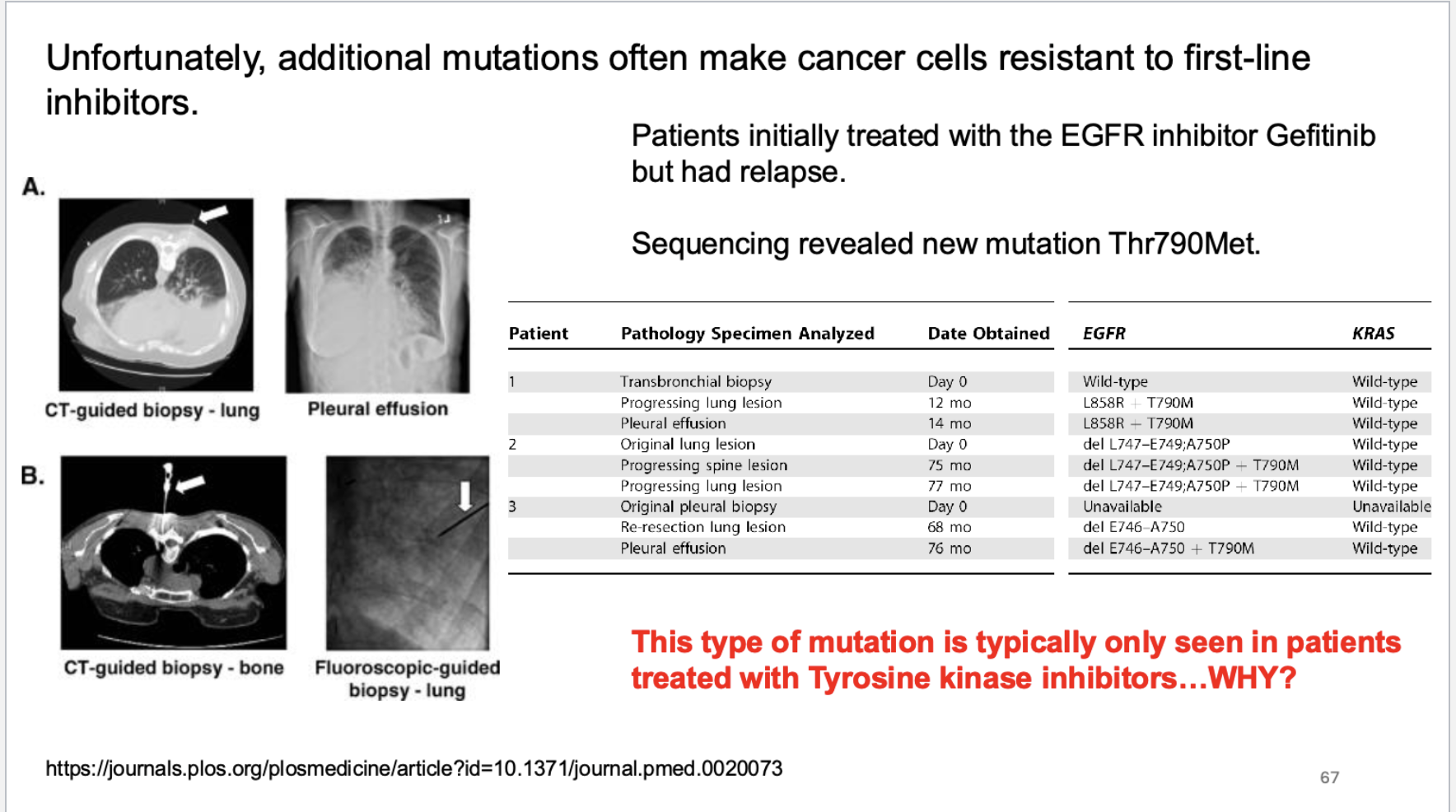
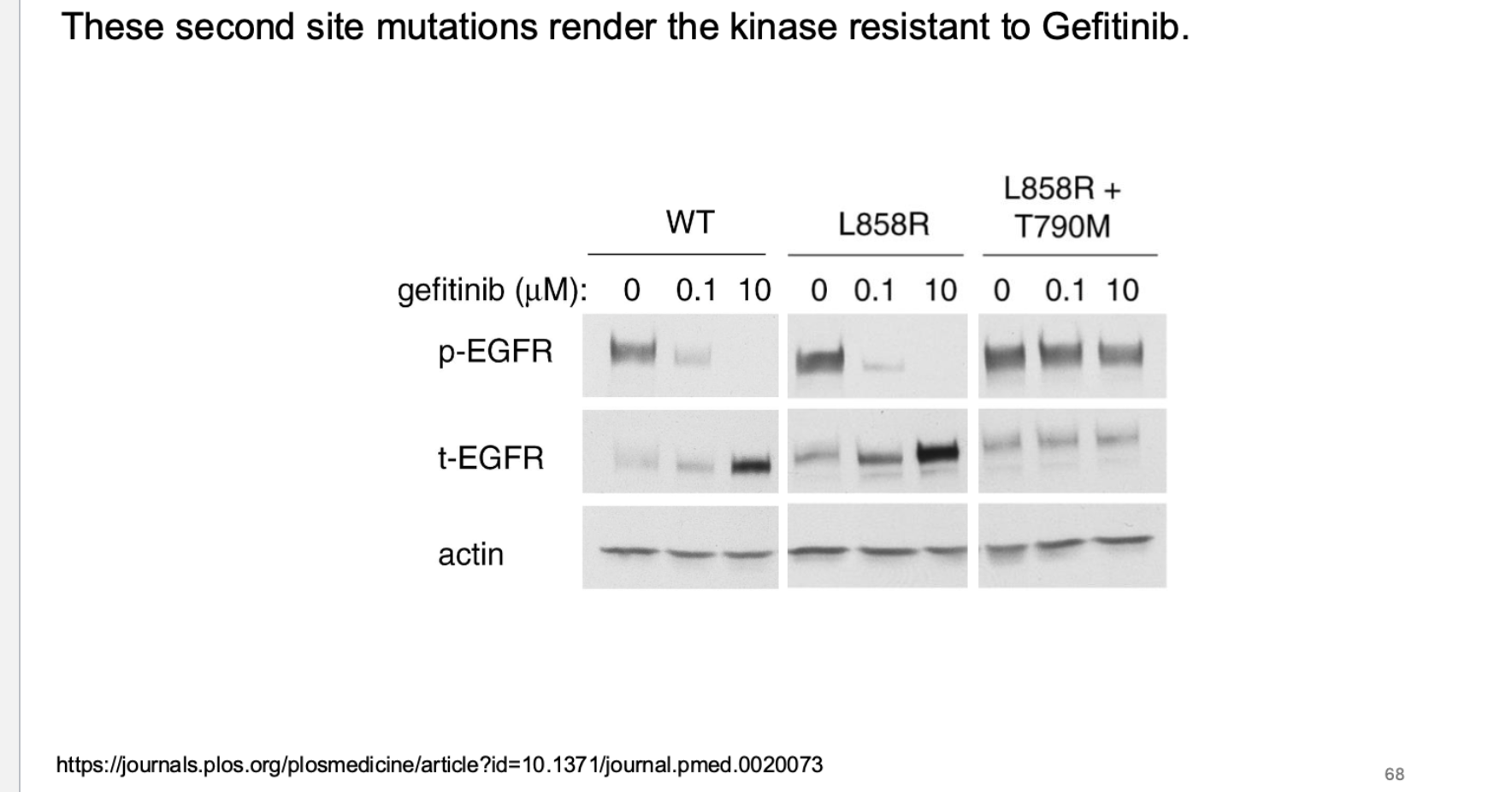
what type of mutations render kinases resistent? what is the nature of these mutations?
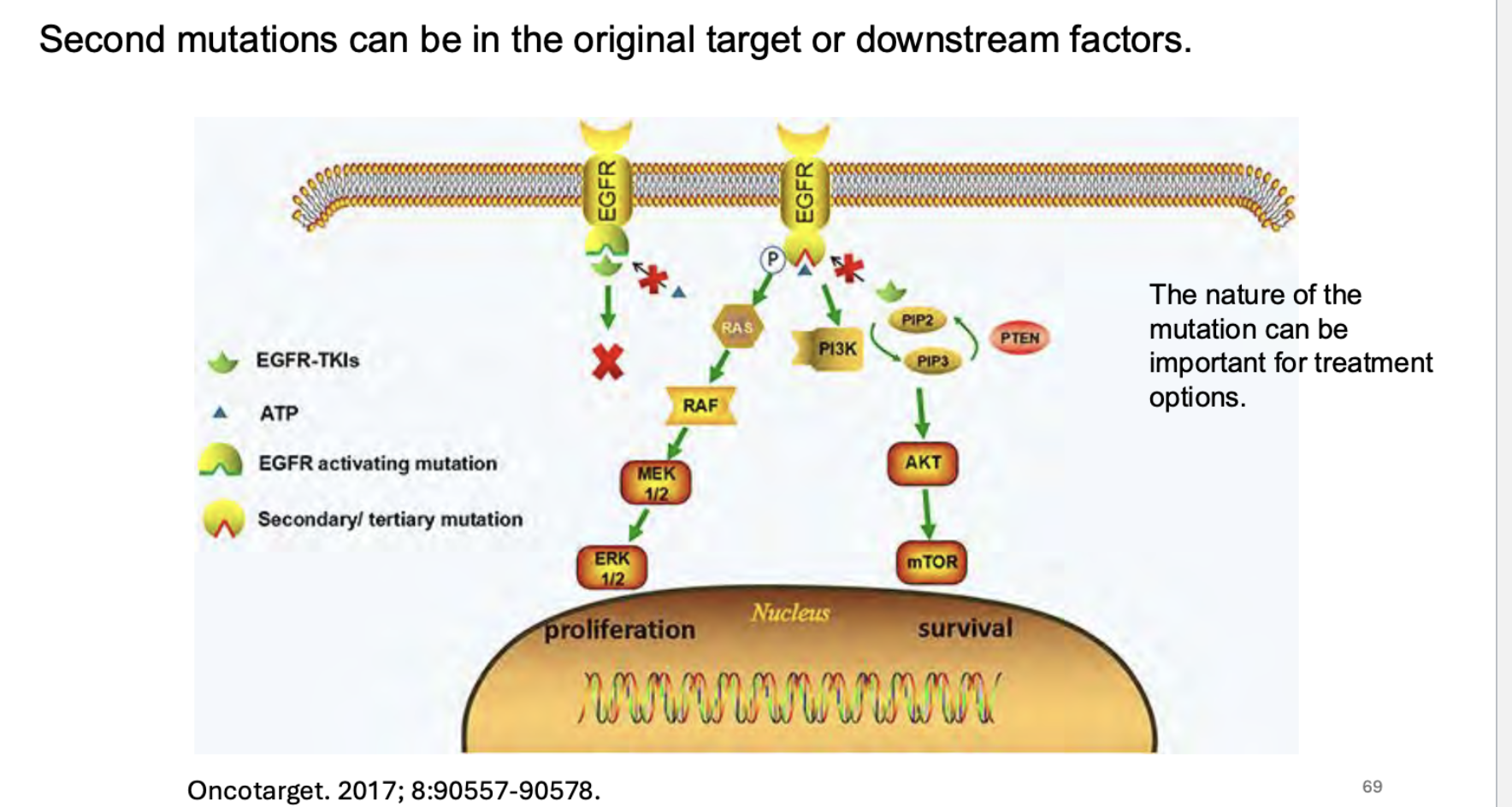
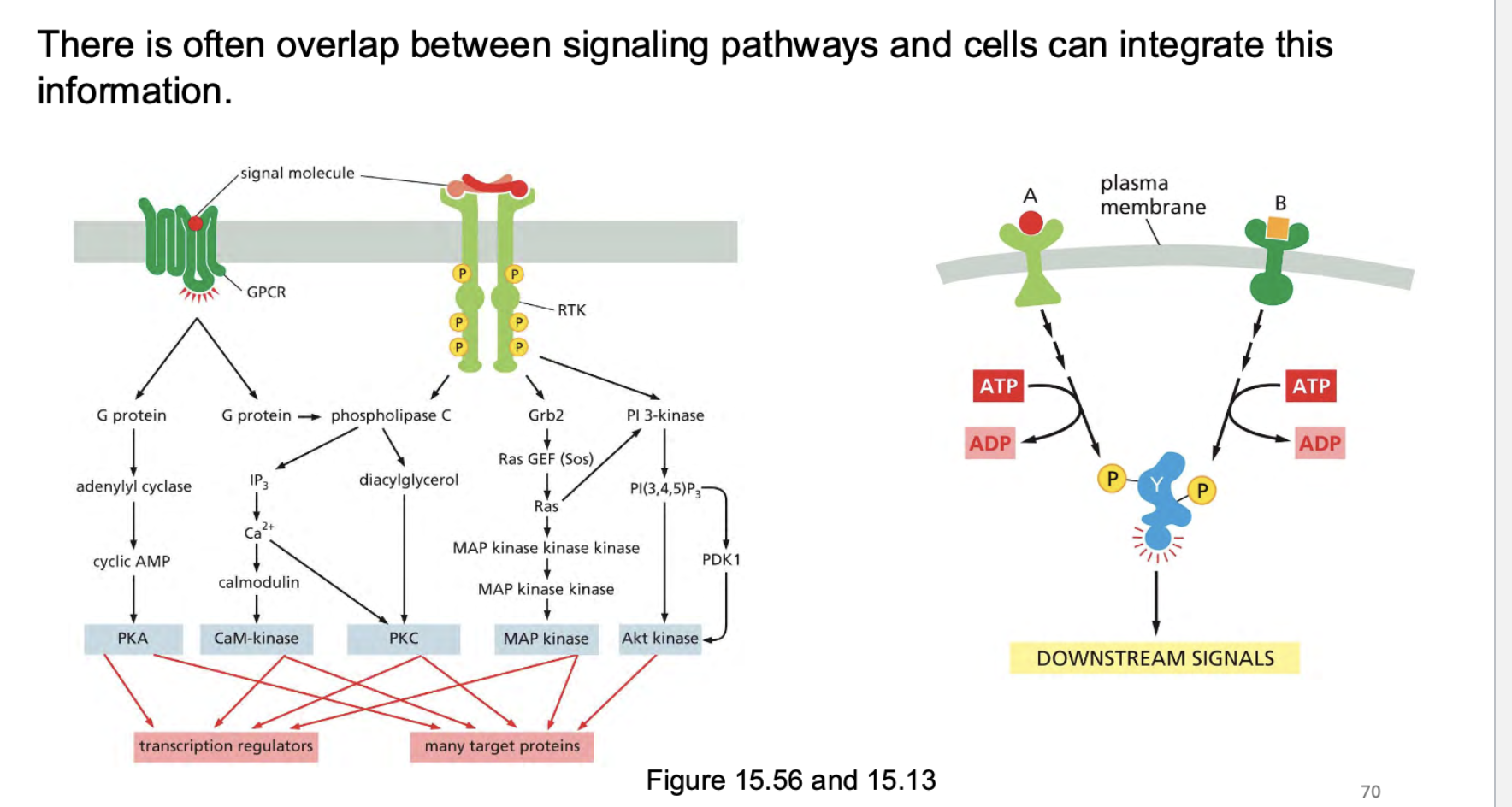
what can happen between signaling pathways and what do cells do?
how do we know how rtk’s work? What kind of experiments can we conduct>?
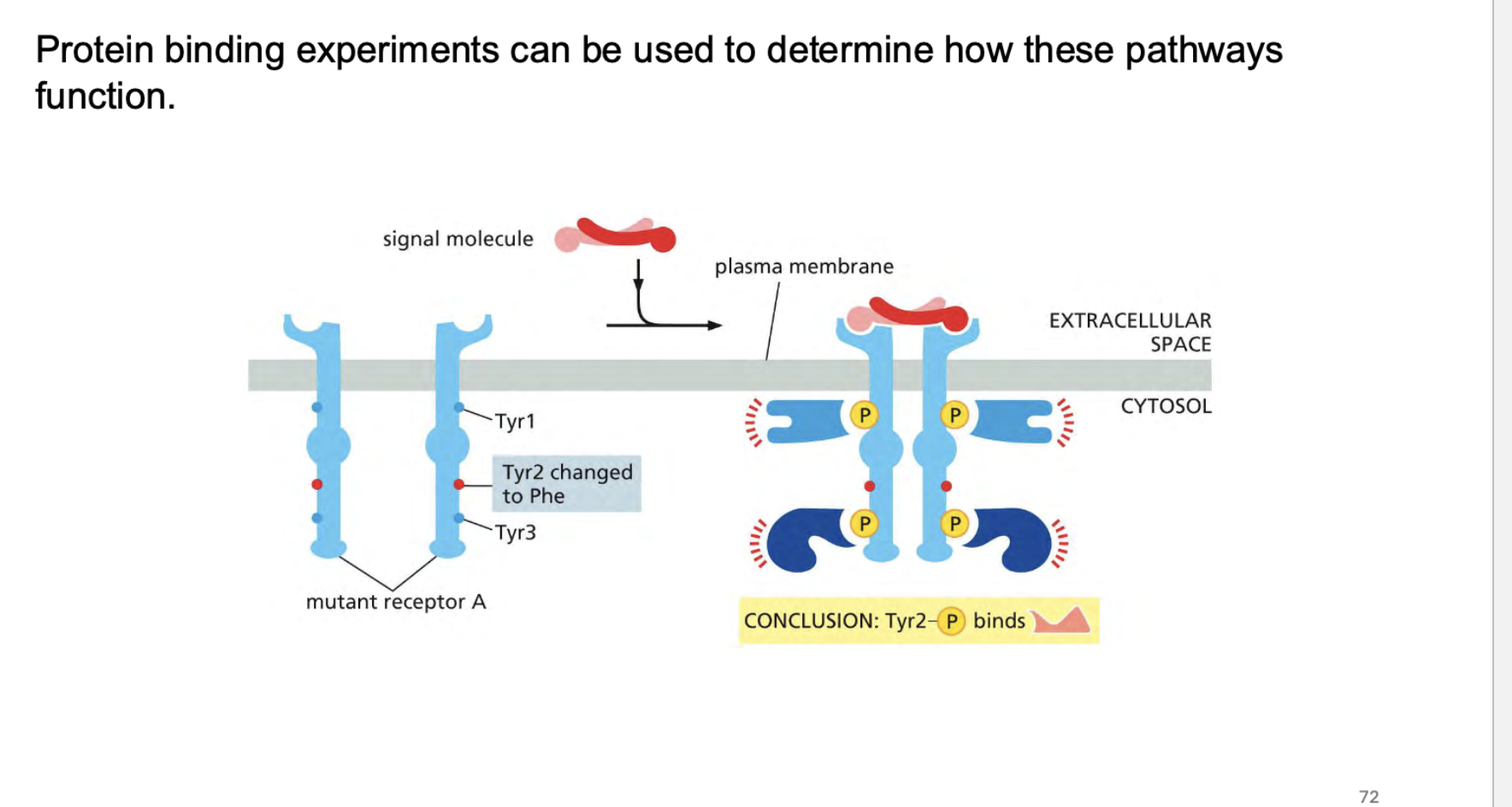
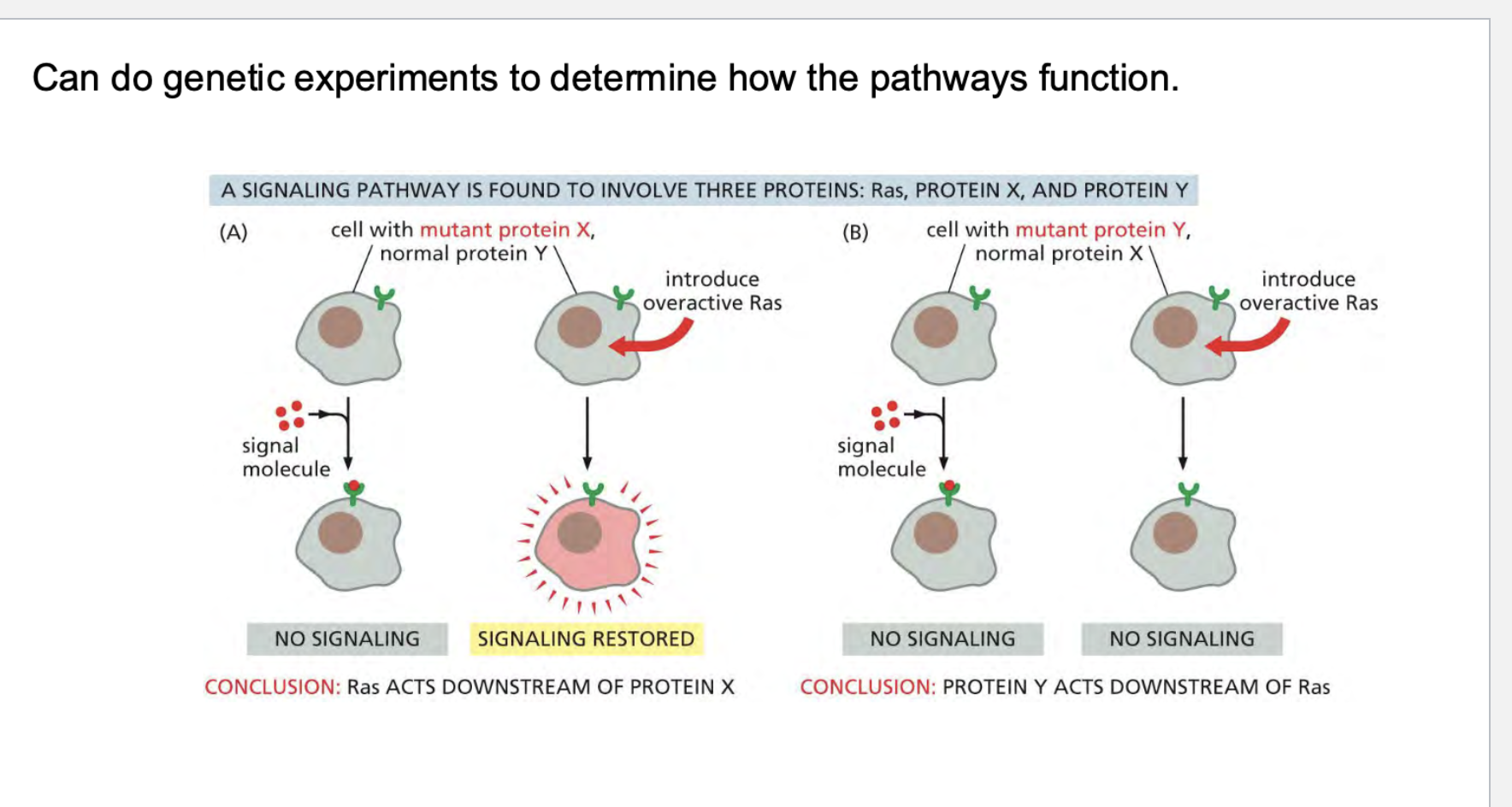
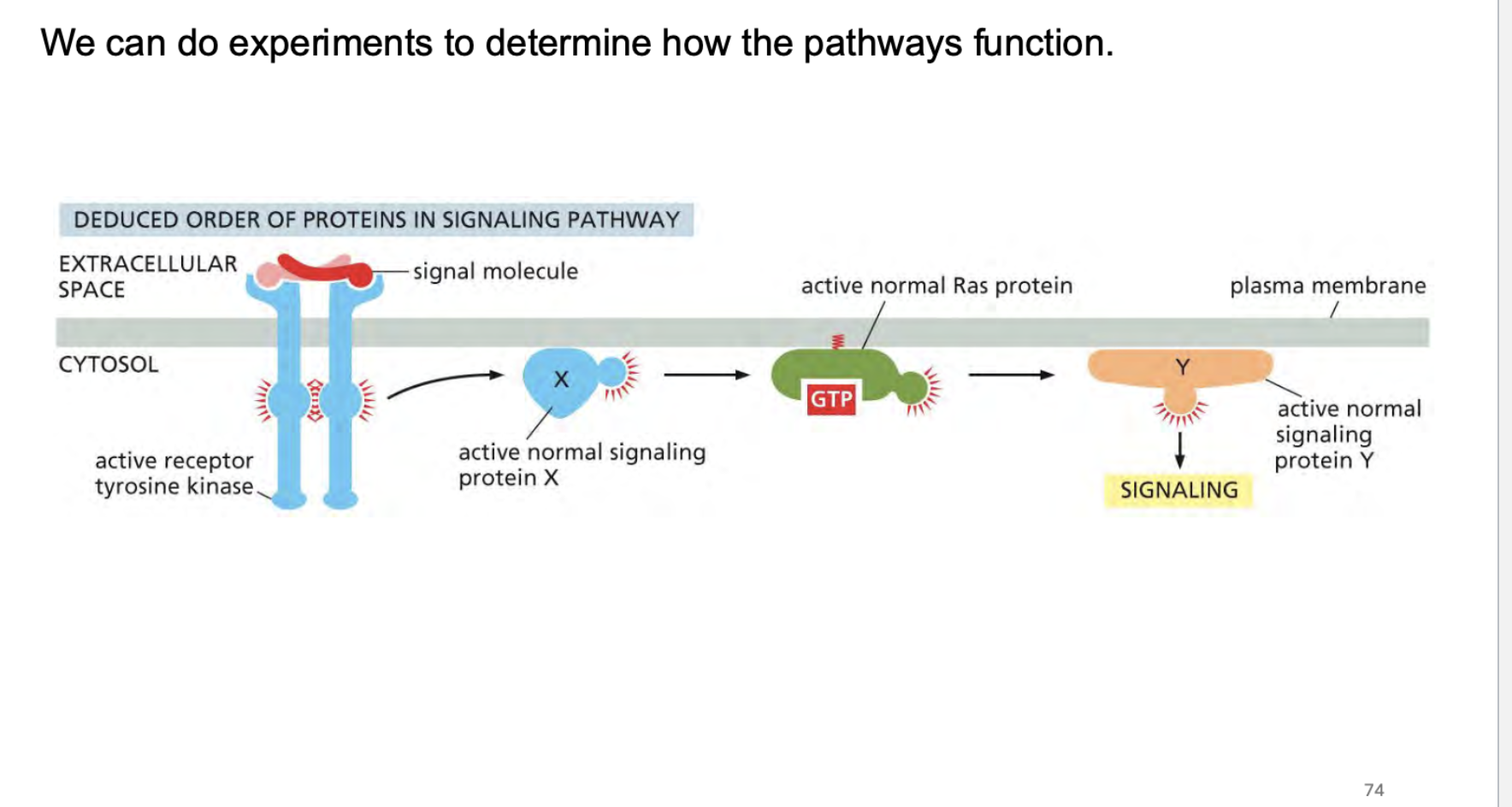
how do some receptors work? These are a little different from enzymes
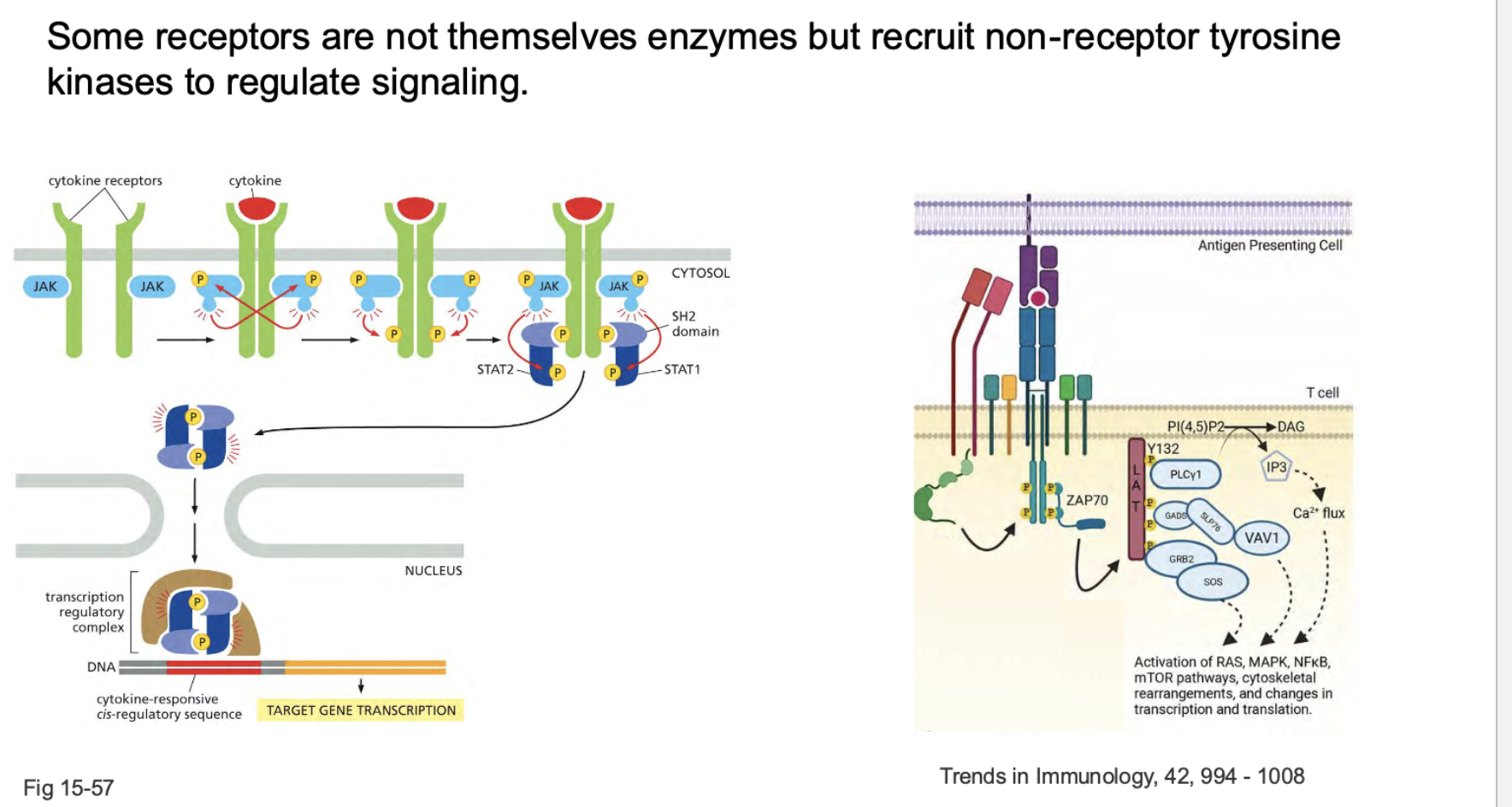
Cytokine ➔ Receptor ➔ JAK activation ➔ Receptor phosphorylation ➔ STAT recruitment ➔ STAT phosphorylation ➔ STAT dimerization ➔ Gene activation
what are cell ecm and cell cell interactions regulated by?
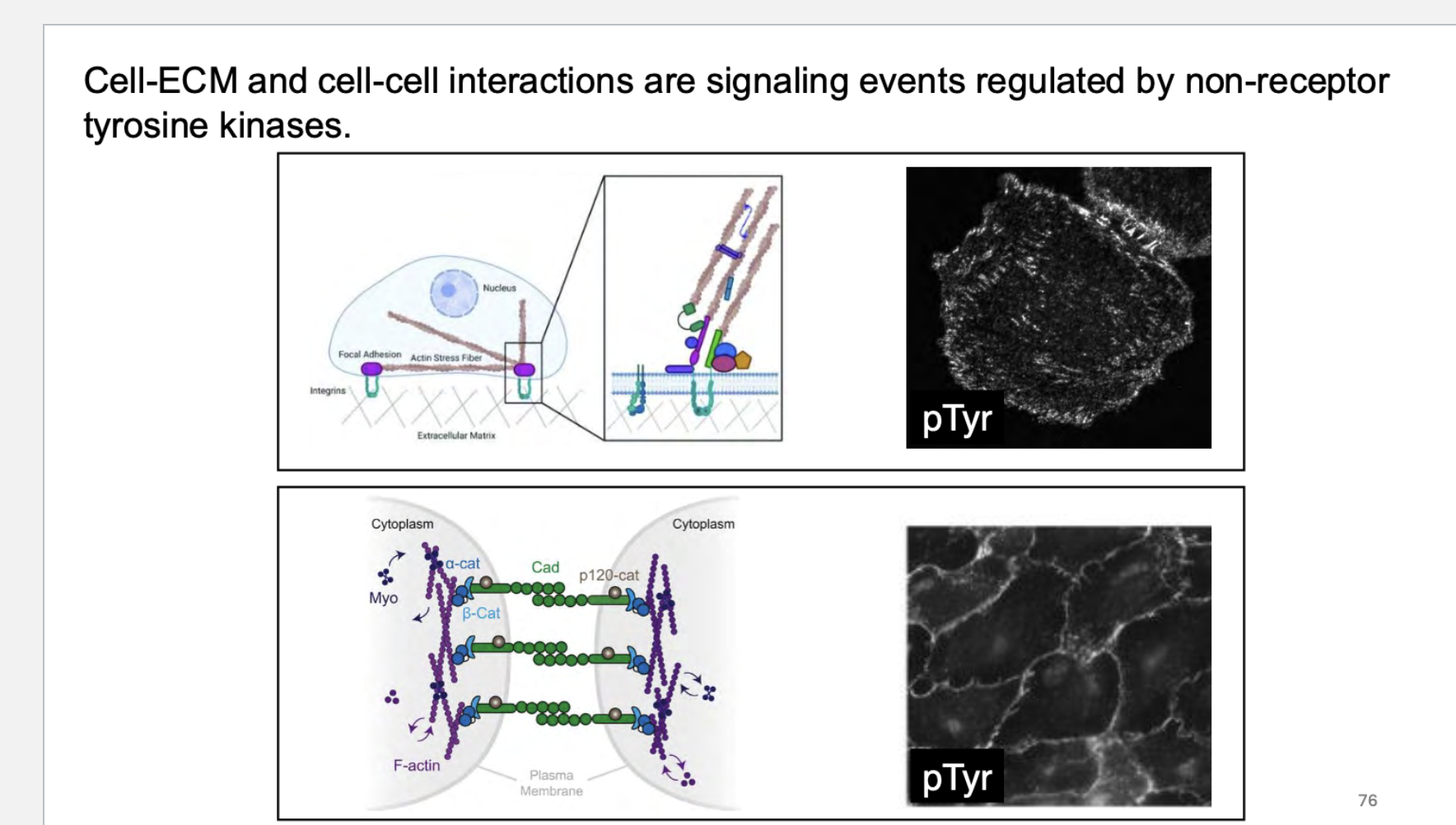
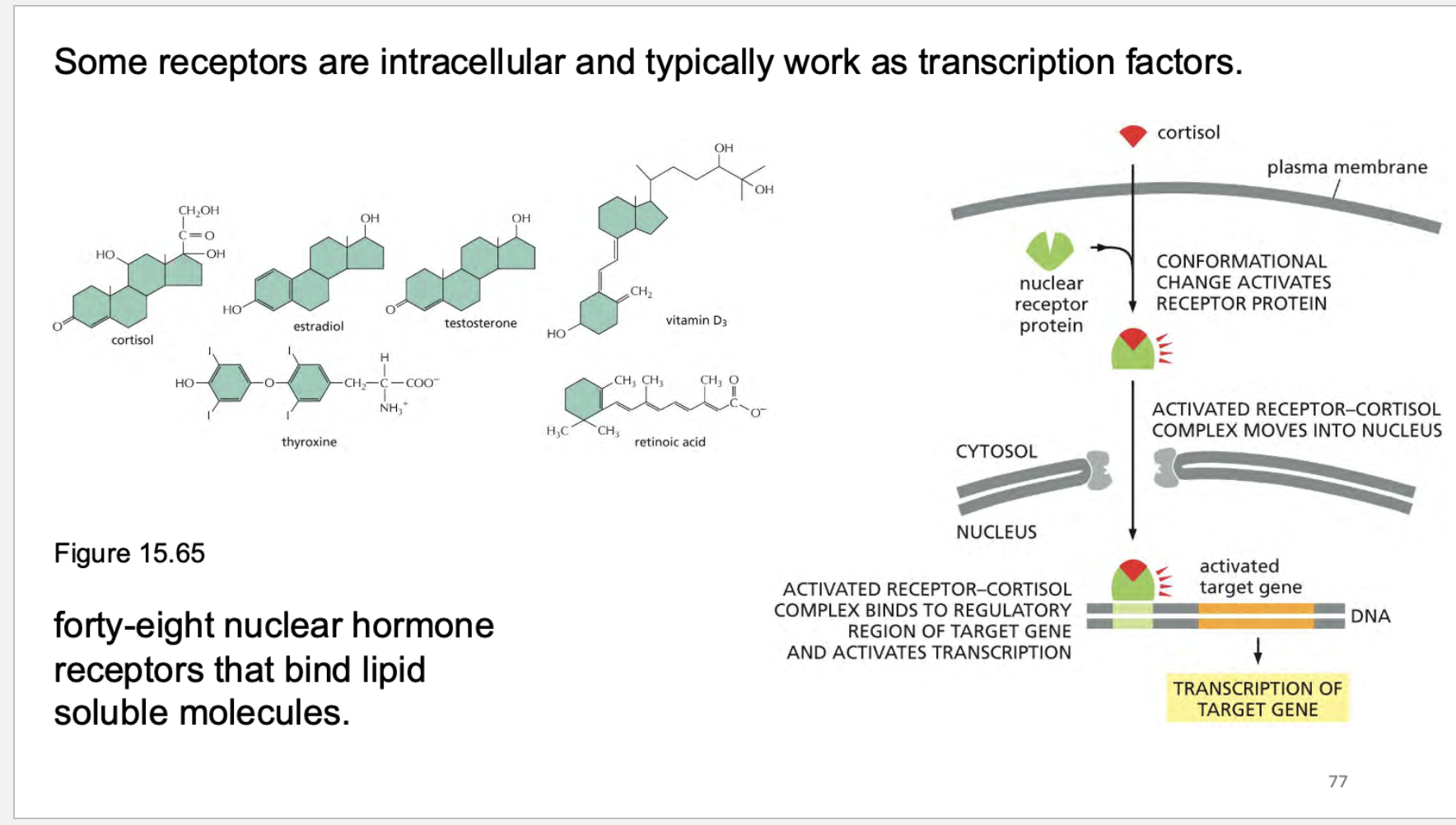
some receptors are _____ and work as these type of factors…