Chapter 4: Reactions in Aqueous Solutions
Solutions
Aqueous (aq): a solid dissolved in water.
Solutions: homogeneous mixtures of two or more pure substances.
Solvent: the liquid, present in greatest abundance.
Solutes: all other substances, they are solid.
When water is the solvent, the solution is called an aqueous solution.
Aqueous Solutions
Substances can dissolve in water by different ways:
Ionic compounds dissolve by dissociation, where water surrounds separated ions.
Dissociation: ionic compound, broken up into ions.
Molecular compounds interact with water, but most do NOT dissociate.
Some molecular substances react with water when they dissolve.
All substances dissolve by solvation, surrounding of the solute by solvent.
Electrolytes and Nonelectrolytes
An electrolyte is a substance that dissociates into ions when dissolved in water.
Ionic compounds → will dissociate
A nonelectrolyte may dissolve in water, but it does not dissociate into ions when it does so.
Molecular compounds → do not dissociate
Electrolytes
Electrolytes like to conduct electricity because they are full of ions.
A strong electrolyte dissociates completely when dissolved in water.
Water-soluble ionic compounds
Strong electrolytes are those solutes that exist in solution completely or nearly completely ions.
Essentially all water-soluble ionic compounds (such as NaCl) and a few molecular compounds (such as HCl) are strong
Examples: NaF and CaCl2
A weak electrolyte only dissociates partially when dissolved in water.
Weak acids and bases, partial dissociation, not soluble.
Weak electrolytes are those solutes that exist in solution mostly in the form of neutral molecules with only a small fraction in the forms of ions.
A nonelectrolyte does not dissociate in water.
Acids
Acids are substances that ionize in aqueous solution to form H+.
Acids are made up of H+ and anions.
Strong acids completely dissociate in water; weak acids only partially dissociate.
Because H+ consists of ONLY a proton, acids are often called proton donors.
Bases
Bases are substances that react with, or accept, H+ ions; they increase the concentration of OH- (hydroxide) when dissolved in water.
Substances do NOT have to contain OH- to be a base.
Strong bases dissociate to metal cations and hydroxide anions in water; weak bases only partially react to produce hydroxide anions.
Strong or Weak
Strong Acids | Strong Bases |
Hydrochloric acid (HCl) | LiOH (1A) |
Hydrobromic acid (HBr) | NaOH (1A) |
Hydroiodic acid (HI) | KOH (1A) |
Chloric acid (HClO3) | RbOH (1A) |
Perchloric acid (HClO4) | CsOH (1A) |
Nitric acid (HNO3) | Ca(OH)2 (2A) |
Sulfuric acid (H2SO4) | Sr(OH)2 (2A) |
Ba(OH)2 (2A) |
Any acid not on this list is weak.
Strong bases include Group 1A metal hydroxides (OH) and heavy group 2A metal hydroxides.
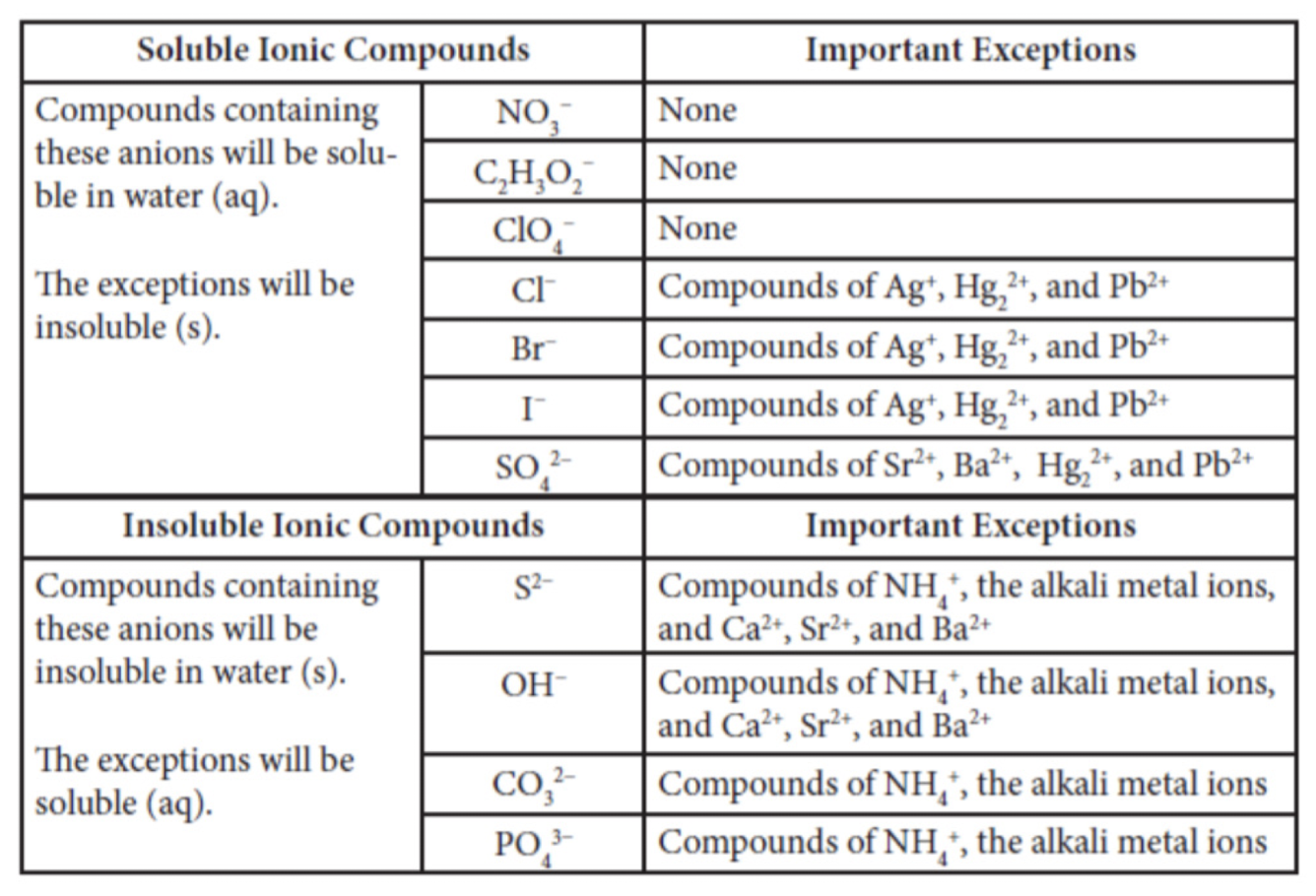
Solubility of Ionic Compounds
Solubility rules only apply to ionic compounds.
Not all ionic compounds dissolve in water.
Examples using the chart:
NaCl → ionic → soluble → strong electrolyte
PbCl2 → ionic → insoluble → weak electrolyte
Ba(NO3)2 → ionic → soluble → strong electrolyte
C2H4 → not acid or base → nonelectrolyte
HF → molecular → acid → weak → weak electrolyte
Precipitation Reactions
Precipitation reactions: occur when two solutions containing soluble salts are mixed and an insoluble salt is produced.
Precipitate: the solid produced from a precipitation reaction.
How to Predict Whether a Precipitate Forms When Strong Electrolytes Are Mixed
Note the ions present in the reactants
Consider the possible cation-anion combinations
Use the solubility rules table to determine if any of the combinations are insoluble
Completing and Balancing Double Replacement Reactions
Use chemical formulas of the reactants to determine which ions are present.
Write formulas for the products: cation from one reactant, anion from the other. Use charges to write proper subscripts.
Check solubility rules. If either product is insoluble, a precipitate forms.
Balance the equation.
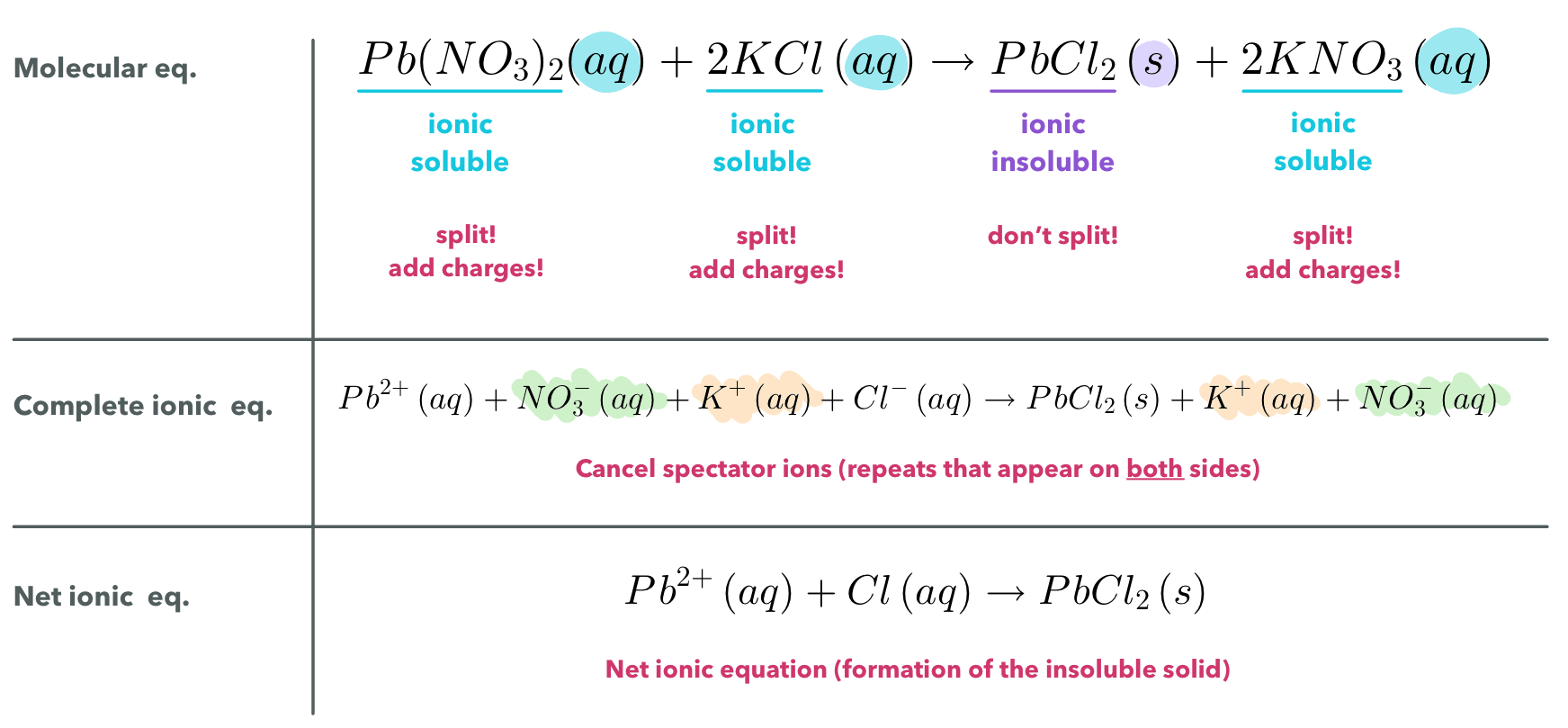
Ways to Write Equations for Double Replacement Reactions
Molecular equation → balance
Complete ionic equation → separate aqueous, include charges
Net ionic equation → cancel spectators, leave charges
Molecular Equation
Molecular equation: lists the reactant and products without indication the ionic nature of the compounds. The only given indication of the overall reaction is the states if matter given.
Complete Ionic Equation
Complete ionic equation: all strong electrolytes (strong acids, strong bases, and soluble ionic salts) are dissociated into their ions.
This more accurately reflects the species that are found in the reaction mixture
Net Ionic Equation
To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right.
Spectator ions: the ions that are crossed out.
The remaining ions are the reactants that form from the product - an insoluble salt in a precipitation reaction.
How to write a net ionic equation
Write a balanced molecular equation for the reaction.
Rewrite the equation to show the ions that form in solution when each soluble strong electrolyte dissociates into its ions. Only strong electrolytes dissolved in aqueous solution are written in ionic form.
Identify and cancel spectator ions.
Writing equations
Example: Use the molecular equation to cancel spectator ions and write the net ionic equation: Pb(NO_{3})(aq)+2KCl(aq) → PbCl_{2}(aq)+2KNO_{3}(aq)
Neutralization Reactions
Neutralization reactions: reactions between an acid and a base.
Specific type of double replacement reactions.
When the base is a metal hydroxide, water and a salt (an ionic compound) are produced.
These equations can be written as molecular, complete ionic, or net ionic equations.
HCl (aq) + NaOH (aq) → HO (l) + Na2Cl (aq)
acid + base → water + salt
Oxidation-Reduction Reactions
Oxidation: the loss of electrons
Reduction: the gain of electrons
One can not occur without the other.
The reactions are often called redox reactions.
OIL RIG
Oxidation Is Loss
Reduction Is Gain
Oxidation Numbers
To determine if an oxidation-reduction reaction has occurred, we assign an oxidation number to each element in a neutral compound or charged entity.
This is a “bookkeeping” method - it does NOT imply that the atoms have these charges.
Rules to Assign Oxidation Numbers
1.) Atoms in their elemental form have an oxidation number of zero.
Fe (s) = 0
Sn (s) = 0
Cu (s) = 0
Diatomics
N2 = 0
Br2 = 0
2.) The oxidation number of a monatomic ion (ions with multiple charges) is the SAME as its ionic charge.
Cu2+ = +2
Cu3+ = +3
Fe2+ = +2
3.) Nonmetals usually have negative oxidation numbers, although they sometimes can be positive.
Oxygen: usually -2 oxidation number (except in the peroxide ion, where it is -1).
Peroxide = H2O2
H = +1
O = -1
Hydrogen: usually +1 oxidation number when bonded to a nonmetal and -1 when bonded to a metal.
Fluorine: -1
Other halogens (7A) : usually -1, unless combined with oxygen (oxyanions), where they will be positive.
4.) The sum of the oxidation numbers in a neutral compound is zero; the sum of the oxidation numbers in a polyatomic ion is the charge on the ion.
Remember to count EVERY atom, no matter how large the subscript, when assigning oxidation numbers.
Peroxide = H2O2
H = +1
O = -1
2(1) + 2(-1) = 0
Balancing Redox Reactions
Displacement Reactions
Single replacement reaction
In displacement reactions, ions oxidize an element (H+ oxidizes Mg below)
The ion is displaced (replaced) in solution (Mg replaces H+ below)
Activity Series and Hydrogen
The elements above hydrogen will react with acids to produce hydrogen gas.
Elements below will NOT react!
A reactive metal is oxidized to a cation.
The stronger the metal, the harder it is to switch in displacement.
Top part has the most reactive metals.
Bottom part has the least reactive metals
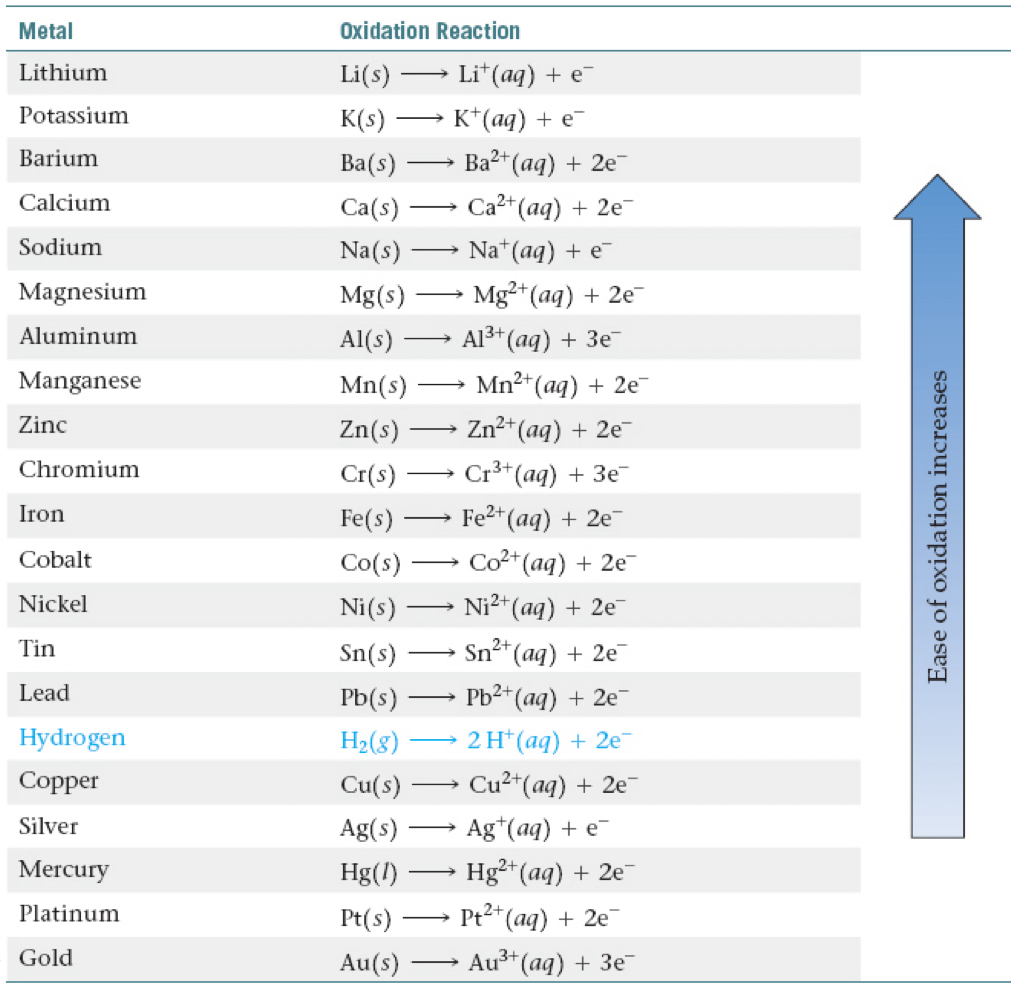
Metal/Acid Displacement Reactions
Elements higher on the activity series are more reactive.
They will exist as ions.
The element below will exist as the element.
Example: 2AgNO3 (aq) + Cu (s) → Cu(NO3)2 (aq) + 2Ag (s)
Copper is stronger than silver, so it can replace Ag.
This reaction can occur.
Molarity
The quantity of solute in a solution can matter to a chemist.
Concentration: the amount dissolved.
Molarity: a way to measure the concentration of a solution.
Equation: Molarity (M) = \dfrac{moles\:of\:solute}{volume\:of\:solute\:in\:Liters} or \dfrac{n}{v}
moles of solute = solid that you are dissolving into liquid
volume of solution is in liters
n = moles
v = volume in liters
Molarity can be used as a conversion factor between moles and liters.
Dilution
Dilution: when you have made something that is too concentrated, you’re going to have to add more liquid to “water” it down.
A solution can be diluted by adding ONLY solvent.
The concentration is LOWER, but the MOLES don’t change.
The molarity of the new solution can be determined from the equation (both are the same):
M_{1}V_{1}=M_{2}V_{2}
M1 and M2 are the molarity of the concentrated and dilute solutions.
V1 and V2 are the volumes of the two solutions
M_{c}V_{c}=M_{d}V_{d}
c = concentrated
d = dilute
Before = after
Remember: M x V (in L) = moles
Reference: Chemistry The Central Science (14th Edition)