4: Biomolecules
Organic Molecules
Organic — used to describe compounds that contain carbon, specifically C—C or C—H bonds.
Four Major Groups of Organic Substances
Carbohydrates
Lipids
Proteins
Nucleic acids and related molecules
Functional Groups — used to describe certain arrangements of atoms attached to the carbon core of many organic molecules.
Also called radicals.
Organic radicals are often designated simply as R .
Free Radical — a functional group that is temporarily unattached and is highly reactive because of unpaired electrons.
Principal Functional Chemical Groups

Carbohydrates
All carbohydrate compounds contain the elements carbon, hydrogen, and oxygen—usually in the ratio of 1 to 2 to 1.
Carbohydrates include the substances commonly called sugars and starches.
It provides the primary source of chemical energy needed by every body cell.
It serves as the structural role for RNA and DNA.
FUNCTION | EXAMPLE |
Energy | Simple sugars provide the main source of energy for cells; complex carbohydrates may provide temporary energy storage |
Molecular structure | Ribose and deoxyribose sugars serve as components of RNA and DNA subunits |
Cell membrane components | Sugars on cell membranes may act as signals or identification tags, as in immune system identification of cell types |
Extracellular matrix | Carbohydrates make up important functional materials within the substance found between cells of some tissues |
Dietary fibre | Carbohydrates that make up plant fibres promote digestive health |
Types of Carbohydrates
Monosaccharides | Disaccharides | Polysaccharides |
Simplest form of carbohydrates, consisting of one sugar unit. | Carbohydrates formed by two monosaccharides linked together. | Complex carbohydrates formed by many monosaccharide units |
Smallest, simple sugars. | Larger, composed of two monosaccharides | Largest, made of long chains of monosaccharides |
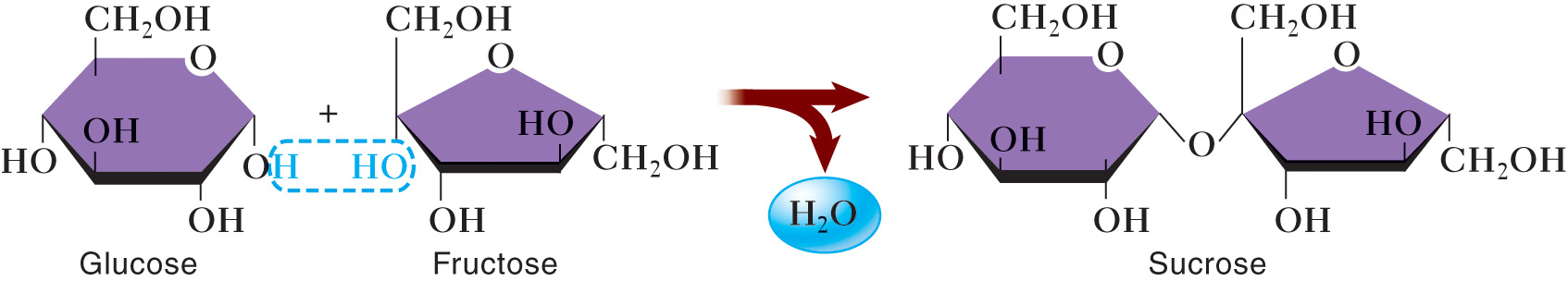
Glucose, fructose, galactose, ribose, deoxyribose | Sucrose (table sugar), lactose (milk sugar), maltose | Glycogen, starch, cellulose |
Single sugar molecule, can be linear or ring-shaped | Two sugar molecules linked via dehydration synthesis | Long chains (linear or branched) of sugar molecules |
Quick source of energy; building blocks for larger carbohydrates | Source of energy, easily broken down into monosaccharides | Energy storage (glycogen in animals, starch in plants) and structural support (cellulose in plants). |
Water-soluble (polar). | Water-soluble but less so than monosaccharides | Generally insoluble or less soluble in water |
Found in fruits, vegetables, and honey (e.g., glucose). | Found in milk (lactose), sugarcane (sucrose), grains (maltose) | Found in plants (starch, cellulose), animals (glycogen). |
Rapid energy release due to quick digestion | Broken down into monosaccharides for energy release | Provides sustained energy when broken down |
No bonding, single units | Linked by glycosidic bonds formed during dehydration synthesis | Long chains linked by glycosidic bonds |

Glucose — the most important simple sugar; a six-carbon sugar with the formula C 6 H 12 O 6.
It is present in the dry state as a straight chain but curls into a cyclic compound (ring) when dissolved in water.
Polymer — any large molecule made up of many identical small molecules.
Glycogen — a polymer of glucose, is sometimes referred to as animal starch; the main polysaccharide in the body and has an estimated molecular weight of several million.
Lipids
Lipids — these are water-insoluble organic biomolecules, as they are non-polar.
Most lipids, many with an oil-like consistency and greasy feel, dissolve readily in some organic solvents such as ether, alcohol, or benzene.
The proportion of oxygen in lipids is much lower than that in carbohydrates.
Many are used for energy purposes, whereas others serve a structural role and function as integral parts of cell membranes.
It also serve as vitamins or protect vital organs by serving as “fat pads”, or shock absorbers.
Major Functions of Human Lipid Compounds
FUNCTION | EXAMPLE |
Energy | Lipids can be stored and broken down later for energy; they yield more energy per unit of weight than carbohydrates or proteins do. |
Structure | Phospholipids and cholesterol are required components of cell membranes. |
Vitamins | Lipid-soluble vitamins: vitamin A forms retinal (necessary for night vision); vitamin D increases calcium uptake; vitamin E promotes wound healing; and vitamin K is required for the synthesis of blood-clotting proteins. |
Protection | Fatty tissue surrounds and protects organs. |
Insulation | Fatty tissue under the skin minimizes heat loss; lipid tissue (containing myelin) covers nerve cells and electrically insulates them. |
Regulation | Steroid hormones regulate many physiological processes; for example, oestrogen and testosterone are responsible for many of the differences between females and males; prostaglandins help regulate inflammation and tissue repair; some phospholipids regulate cell functions. |
Triglycerides
Triglycerides — also known as fats, are the most abundant lipids, and they function as the body’s most concentrated source of energy.
Two types of building blocks are needed to synthesize or build a fat molecule:
Glycerol — forms the backbone of triglycerides by bonding with three fatty acids.
Fatty Acids — the building blocks of the fat in our bodies and in the food we eat.
Types of Fatty Acids
Saturated Fatty Acids | Monounsaturated Fatty Acids | Polyunsaturated Fatty Acids |
All carbon atoms are fully saturated with hydrogen; no double bonds | One double bond in the hydrocarbon chain | Two or more double bonds in the hydrocarbon chain |
Straight chain, no kinks or bends | Contains one kink/bend due to a single double bond | Multiple kinks/bends due to multiple double bonds |
Palmitic acid, stearic acid (found in animal fats, butter). | Oleic acid (found in olive oil, avocados, and nuts) | Linoleic acid, alpha-linolenic acid (found in fish oil, flaxseed, sunflower oil) |
Solid (due to tight molecular packing). | Typically liquid, may be semi-solid (e.g., olive oil). | Liquid (due to less dense molecular packing). |
Mainly from animal fats (lard, butter, dairy), some tropical oils (coconut, palm oil). | Plant oils (olive oil, canola oil), nuts, avocados. | Vegetable oils (soybean, corn, sunflower), fatty fish, flaxseed. |
Excess consumption linked to increased risk of heart disease and raised LDL cholesterol levels. | Generally considered heart-healthy, can lower bad cholesterol (LDL) and raise good cholesterol (HDL). | Beneficial for heart health, helps lower cholesterol and reduce inflammation. |
More chemically stable, less prone to oxidation and rancidity. | Less stable than saturated fats but more stable than polyunsaturated fats. | Least stable, prone to oxidation and rancidity due to double bonds. |
Provides energy, used for insulation and protection, but too much can lead to fat buildup in arteries | Plays a role in reducing inflammation, maintains cell membranes, and supports heart health | Essential for brain function, cell growth, reducing inflammation, and heart health |
Formation of triglycerides
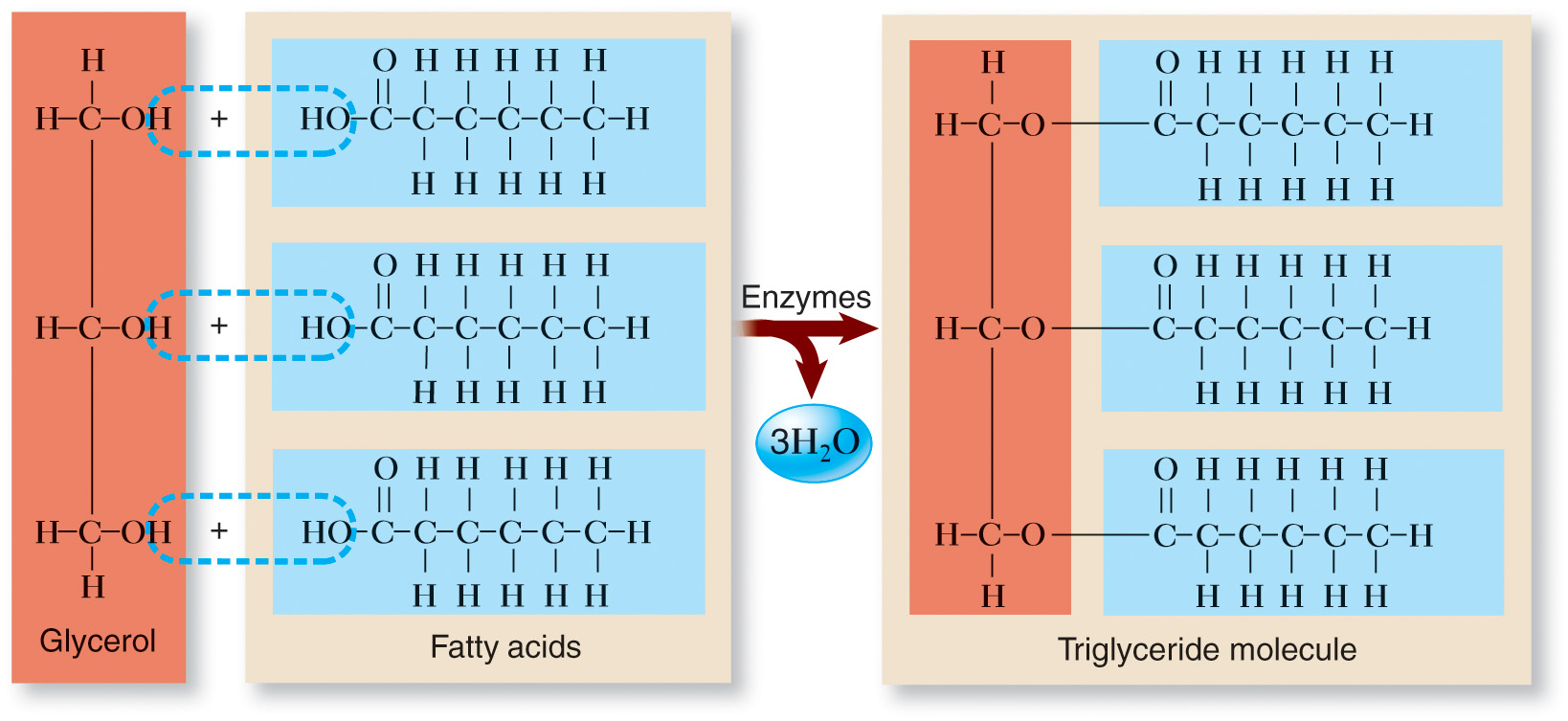
Triglycerides are made up of a glycerol molecule and three fatty acid molecules.
The fatty acids attach to the glycerol through a dehydration synthesis reaction, where water is removed as a byproduct.
Triglyceride Formation:
Building blocks: Glycerol (3-carbon alcohol) and 3 fatty acids.
Process: Dehydration synthesis (water is removed).
Attachment: Fatty acid carboxyl groups (COOH) to glycerol hydroxyl groups (OH).
Fatty Acid Types:
Short-chain: Example is caproic acid (6 carbons).
Long-chain: Have longer carbon backbones.
Triglyceride Variation:
Can contain the same fatty acid (e.g., glycerol tricaproate) or different fatty acids.
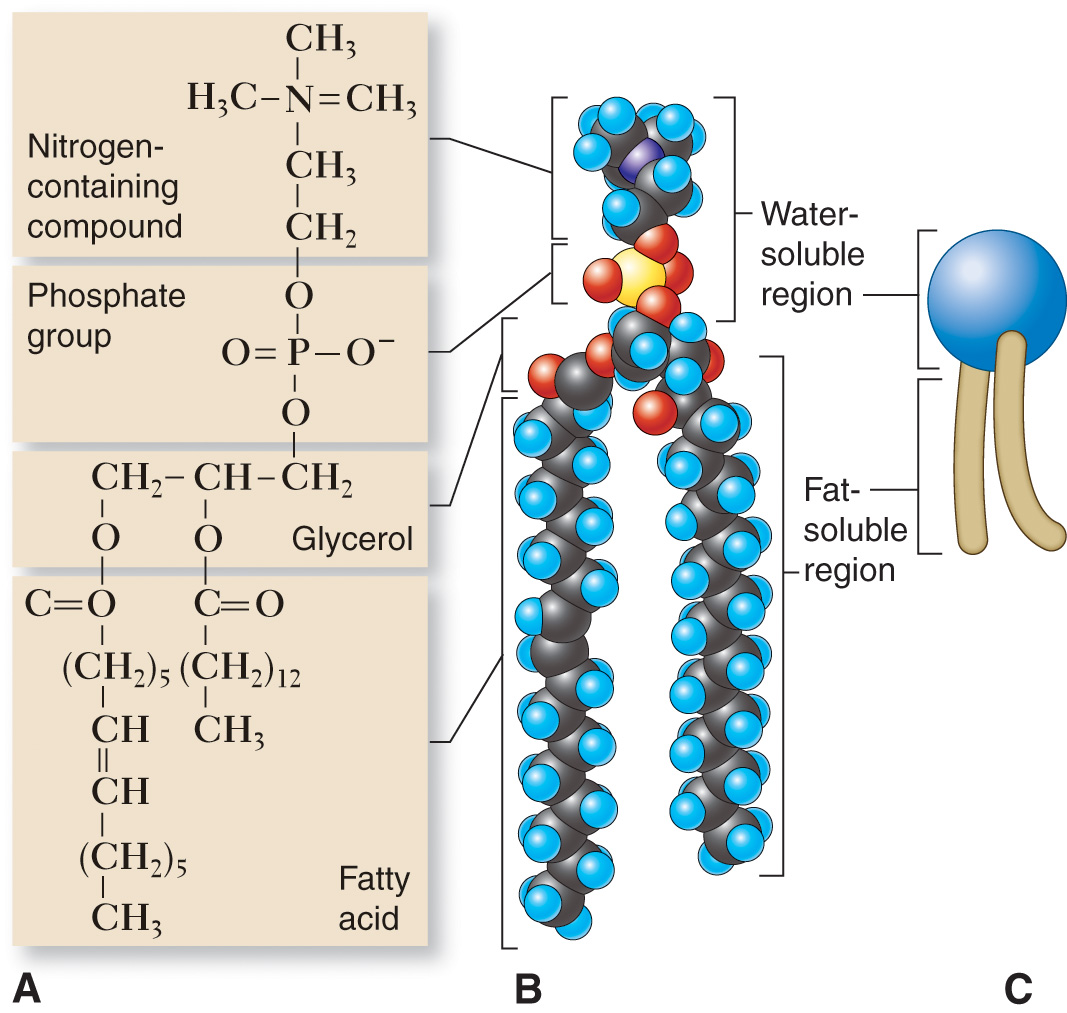
Phospholipids
Phospholipids are lipid compounds that are modified.
In that one of the three fatty acids attached to glycerol in a triglyceride is replaced in a phospholipid by another type of chemical structure containing phosphorus and nitrogen.4
The head, or end of the molecule containing the phosphorus group, in a phospholipid molecule is polar and is therefore water soluble—making it hydrophilic (“water loving”).
The end formed by the two fatty acids is nonpolar and is therefore lipid soluble or hydrophobic (“water fearing”).
Phosphoinositides (PIs) — make up only a small fraction of cellular phospholipids, yet they control almost all aspects of a cell's life and death.
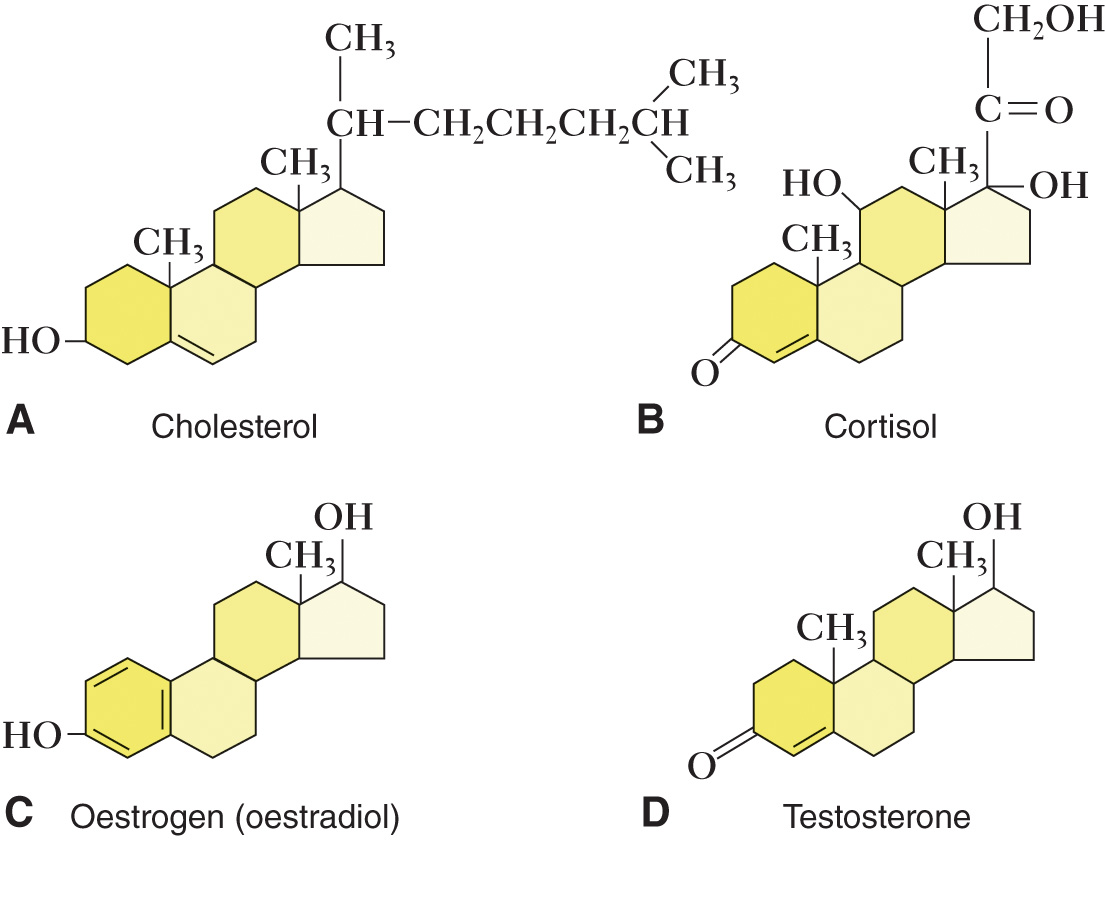
Steroids
Steroids — a large and important class of lipids whose molecules have as their main feature the steroid nucleus.
Its nucleus is composed of four attached rings that are structurally similar but may have widely diverse functions related to the differing functional groups that are attached to them.
The steroid nucleus is also a part of the active hormone form of vitamin D called calcitriol.
Cholesterol — a steroid found in the plasma membrane surrounding every body cell.
The body slightly modifies cholesterol molecules to form such important hormones as cortisone, oestrogen, and testosterone.
It is also used to make the bile salts needed for digestion.
It can travel in the blood only after it has attached to a protein molecule—forming a lipoprotein.
Lipoprotein — these are particles made of protein and fats. They carry cholesterol through bloodstream to the cells.
High-Density Lipoprotein — also known as good cholesterol, it help removes other forms of cholesterol in the bloodstream.
Low-Density Lipoprotein — also known as bad cholesterol, this carries cholesterol to the blood, and helps buildup of cholesterol in arteries.
Mostly associated with atherosclerosis.
Prostaglandins
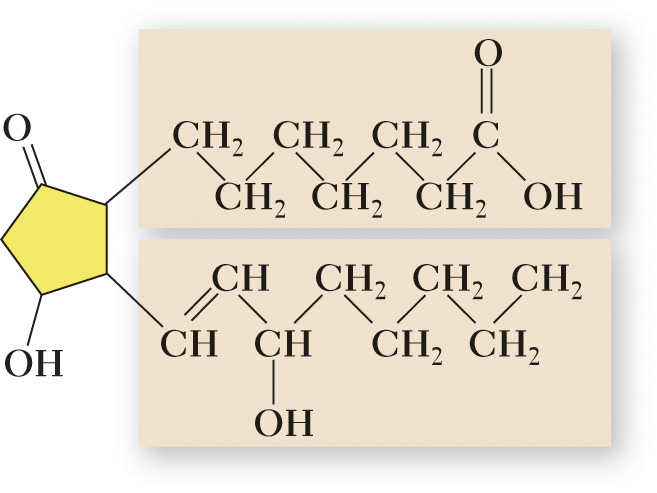
Prostaglandins (PGs) — also known as tissue hormones, are lipids composed of a 20-carbon unsaturated fatty acids that contains a five carbon ring.
They play a crucial role in regulating the effects of several hormones, influence blood pressure and the secretion of digestive juices, enhance the body’s immune system and inflammatory response— and have an important role in blood clotting and respiration.
If a specific type of enzyme, cyclooxygenase (COX), is present to interact with these fatty acids, prostaglandins will be synthesized and released from the cell membrane into the surrounding tissue fluid.
Prostaglandins sometimes serve as inflammatory agents.
They cause local dilation of blood vessels with resulting heat (fever), swelling, redness, and pain.
Prostaglandins sometimes serve to regulate blood clotting.
Aspirin — a commonly used cyclooxygenase (COX) inhibitor that reduces prostaglandin effects in the body such as inflammation, fever, and blood clotting.
Proteins
Protein — the most abundant of the organic compounds in the body.
Four Elements of Proteins
Carbon
Oxygen
Hydrogen
Nitrogen
Structural and Functional Proteins
Structural Proteins | Functional Proteins |
Provide support, strength, and structure to cells and tissues. | Performs specific biological activities, such as catalysing reactions, transporting molecules, or defending the body. |
Maintains cell shape, integrity, contribute to the structure of tissues and organs. | Facilitate and regulate biochemical processes, including metabolism, transport, and defence mechanisms. |
Collagen, keratin, elastin, actin, tubulin. | Enzymes, antibodies, haemoglobin, hormones. |
Generally fibrous, insoluble in water | Typically globular, soluble in water |
Long, rigid, and typically repetitive amino acid sequences forming fibrous or sheet-like structures | Folded into compact, complex shapes that are specific to their function |
FUNCTIONS: - Provides mechanical support to cells and tissues (e.g., collagen in connective tissue). | FUNCTIONS: - Catalyzes biochemical reactions (enzymes). |
Found in connective tissues, skin, hair, nails, muscles, and cytoskeletons. | Found in various body fluids, cells, and within membranes, performing diverse roles in metabolism, immunity, and signaling |
Deficiency in structural proteins like collagen can lead to conditions like osteogenesis imperfecta. | Malfunction in functional proteins like enzymes can lead to metabolic disorders (e.g., phenylketonuria from defective phenylalanine hydroxylase). |
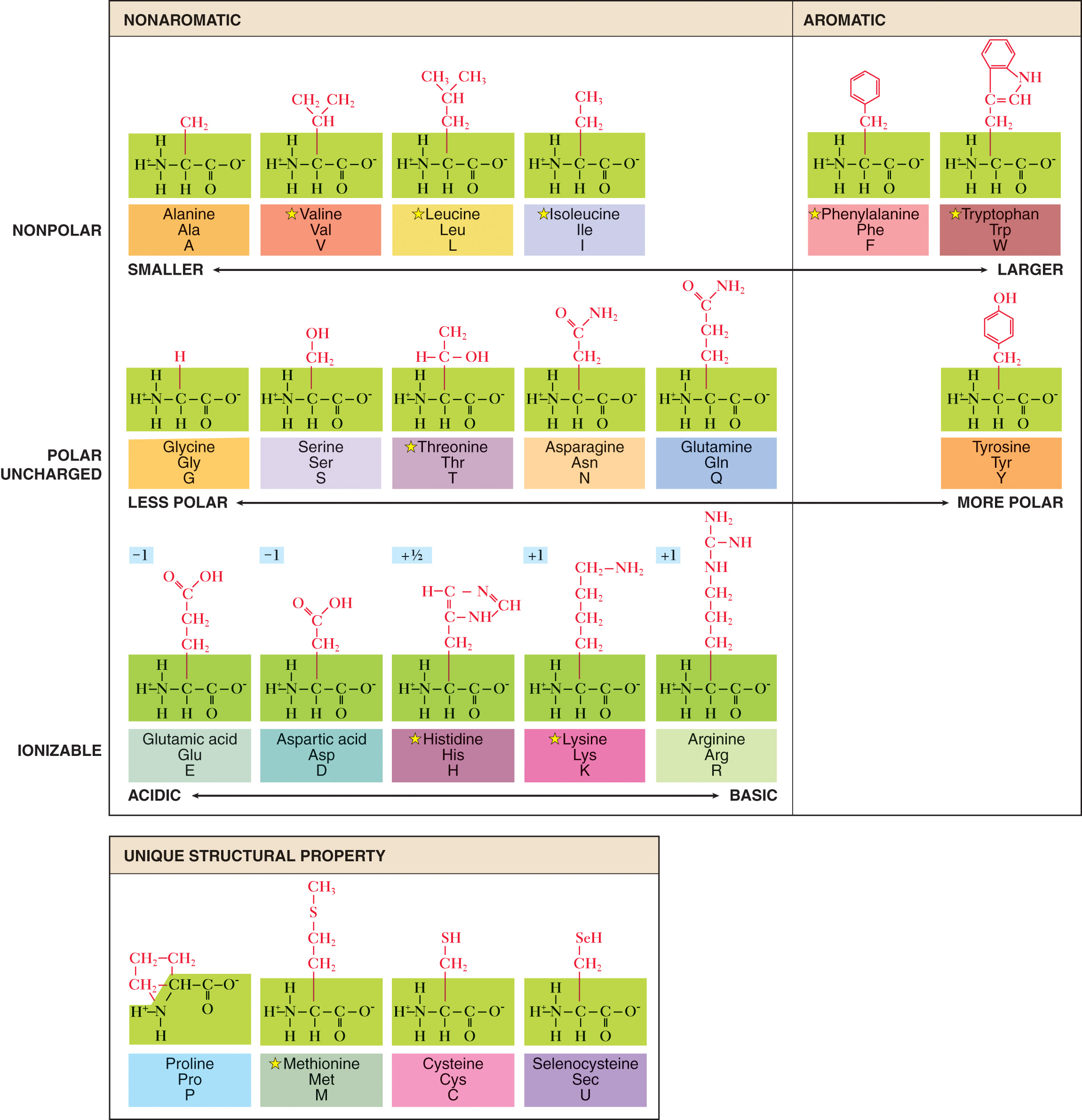
Amino Acids
Amino Acids — formed when the elements that make up a protein molecule are bonded together.
Proteins are composed of 21 naturally occuring amino acids.
8 are known as essential amino acids.
13 are nonessential amino acids.
Basic Structural Formula of an Amino Acid
It consists of a carbon atom (alpha carbon) to which are bonded a positive amino group, a negative carboxyl group, a hydrogen atom, and a functional group (radical R).
The functional group that constitutes the unique, identifying part of an amino acid.
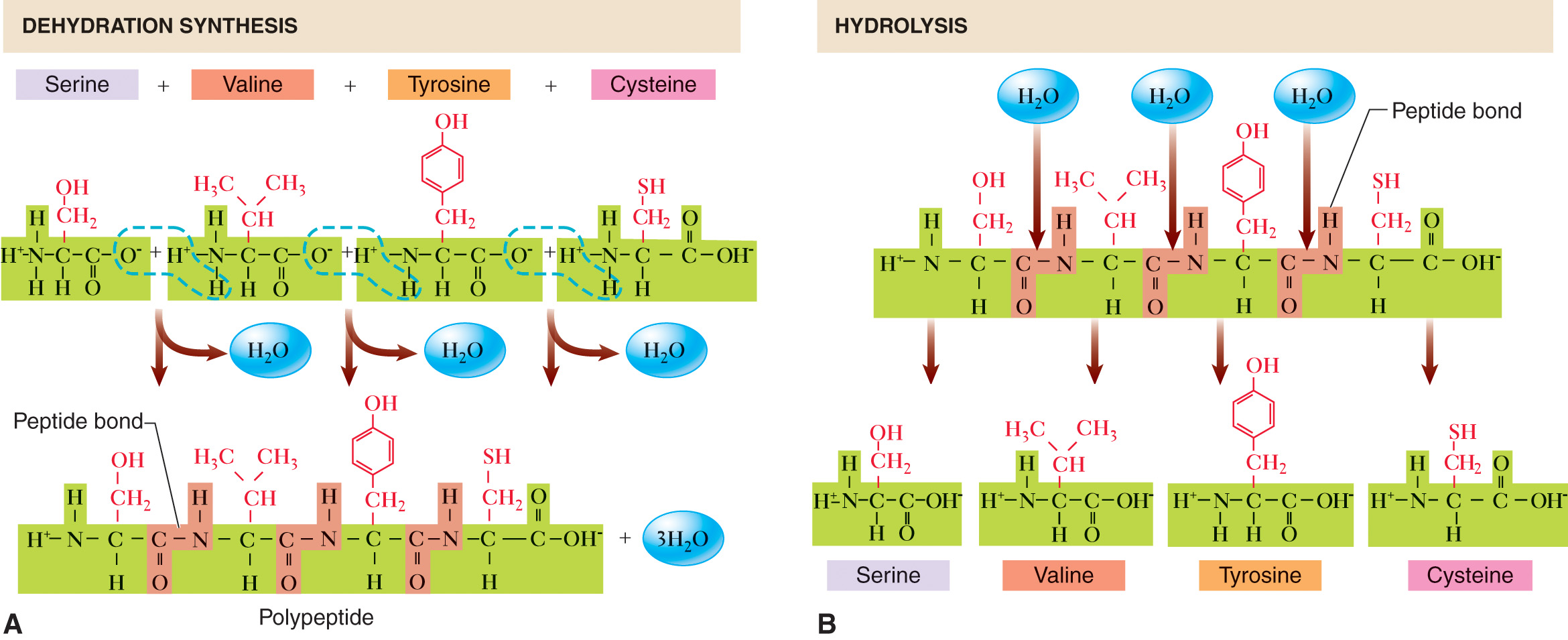
Peptide Bond — one that binds the carboxyl group of one amino acid to the amino group of another amino acid.
Peptide — a compound formed when O from the negative carboxyl group of one amino acid and two H atoms from the positive amino group of another amino acid split off to form water.
Dipeptide — a peptide made up of only two amino acids linked by a peptide bond.
Tripeptide — this consists of three amino acids linked by two bonds.
Polypeptide — a long sequence or chain of amino acids—usually 100 or more—linked by peptide bonds.
When the length of the polymer chain exceeds about 100 amino acids, the molecule is called a protein rather than a polypeptide.

21 amino acids that make up most human proteins
Levels of Protein Structure
Level of Protein Structure | Description | Key Features |
Primary Structure | The sequence of amino acids in a polypeptide chain. | - Linear sequence of amino acids. - Peptide bonds connect amino acids. - Determines the protein's unique characteristics. |
Secondary Structure | Local folding of the polypeptide chain into alpha helices or beta-pleated sheets | - Stabilized by hydrogen bonds. |
Tertiary Structure | The overall 3D shape of a single polypeptide chain, formed by further folding of the secondary structure. | - Complex folding due to interactions between R groups. |
Quaternary Structure | The arrangement of two or more polypeptide chains into a larger, multi-subunit protein complex. | - Multiple polypeptide chains (subunits) combine. |

Other notes:
Parathyroid hormone (PTH) — a protein that retains its primary structure—it is a noodle-like molecule consisting of only one polypeptide chain of 84 amino acids..
Motif — a commonly occurring pattern of alpha helices and/or beta sheets within the secondary structure.
Domains — a tertiary structure may include several complicated “knots”.
Keratin — a threadlike fibrous protein that is rich in the sulphur-containing amino acid cysteine.
Disulphide Linkages — bonds formed when the protein chains in keratin are linked in numerous places by S—S bonds that form between cysteines within each hair shaft.
Chaperones — a group of proteins that acts to direct the steps required for many proteins to fold into the twisted and convoluted shape required for them to function properly.
Chaperonins — oligomeric proteins that assist in the folding of nascent or denatured proteins.
Three Common Types of Protein Models
Ribbon Model — shows the areas where alpha helices and folded sheets form within the molecule.
Space-filling Model — shows each atom as a “cloud” filling up the space occupied by that atom.
Surface-rendering model — shows the three-dimensional boundaries of the whole protein molecule and often colour-coding for charged regions on the surface of the protein.

Importance of protein shape
Properly folded protein molecules are highly organized in their structure and show a very definite relationship between their shape and their function.
Native State — the final, functioning shape for a protein.
Denatured Protein — occurs when a protein loses its normal folded organization and thus loses its functional shape.
Renatured Protein — occurs when the protein shape is restored, resuming its normal function.

Major Functions of Human Protein Compounds
FUNCTION | EXAMPLE |
Provide structure | Structural proteins include keratin of skin, hair, and nails; parts of cell membranes; tendons |
Catalyze chemical reactions | Lactase (enzyme in intestinal digestive juice) catalyzes chemical reaction that changes lactose to glucose and galactose |
Transport substances in blood | Proteins classified as albumins combine with fatty acids to transport them in the form of lipoproteins |
Communicate information to cells | Insulin, a protein hormone, serves as a chemical messenger from islet cells of the pancreas to cells all over the body |
Act as receptors | Binding sites of certain proteins on surfaces of cell membranes serve as receptors for insulin and various other hormones |
Defend body against many harmful agents | Proteins called antibodies or immunoglobulins combine with various harmful agents to render those agents harmless |
Provide energy | Proteins can be metabolized for energy |

Nucleic acids and related molecules
Nucleic Acid — polymers of nucleotides.
Nucleotides — the basic building block of nucleic acids.
Deoxyribonucleotide — consists of the pentose sugar, deoxyribose, a nitrogenous base (either adenine, cytosine, guanine, or thymine), and a phosphate group.
Ribonucleotides — contains the sugar ribose instead of deoxyribose and the nitrogenous base uracil instead of thymine.
DNA and RNA
DNA (Deoxyribonucleic Acid) | RNA (Ribonucleic Acid) |
Double-stranded; forms a double helix | Single-stranded (can fold into complex structures) |
Deoxyribose | Ribose |
Adenine (A), Thymine (T), Guanine (G), Cytosine (C) | Adenine (A), Uracil (U), Guanine (G), Cytosine (C) |
A-T (Adenine with Thymine), G-C (Guanine with Cytosine) | A-U (Adenine with Uracil), G-C (Guanine with Cytosine) |
Primarily in the cell nucleus | Found in both the nucleus and cytoplasm |
Stores genetic information and passes it from generation to generation | Acts as a temporary copy of DNA for protein synthesis and regulation |
Large, long polymers (millions of nucleotides) | Smaller, shorter molecules (varies in size) |
Only one type | Multiple types: messenger RNA (mRNA), transfer RNA (tRNA), ribosomal RNA (rRNA), etc. |
Provides the genetic code (master blueprint) | Translates the genetic code into proteins (mRNA, tRNA, rRNA involved) |
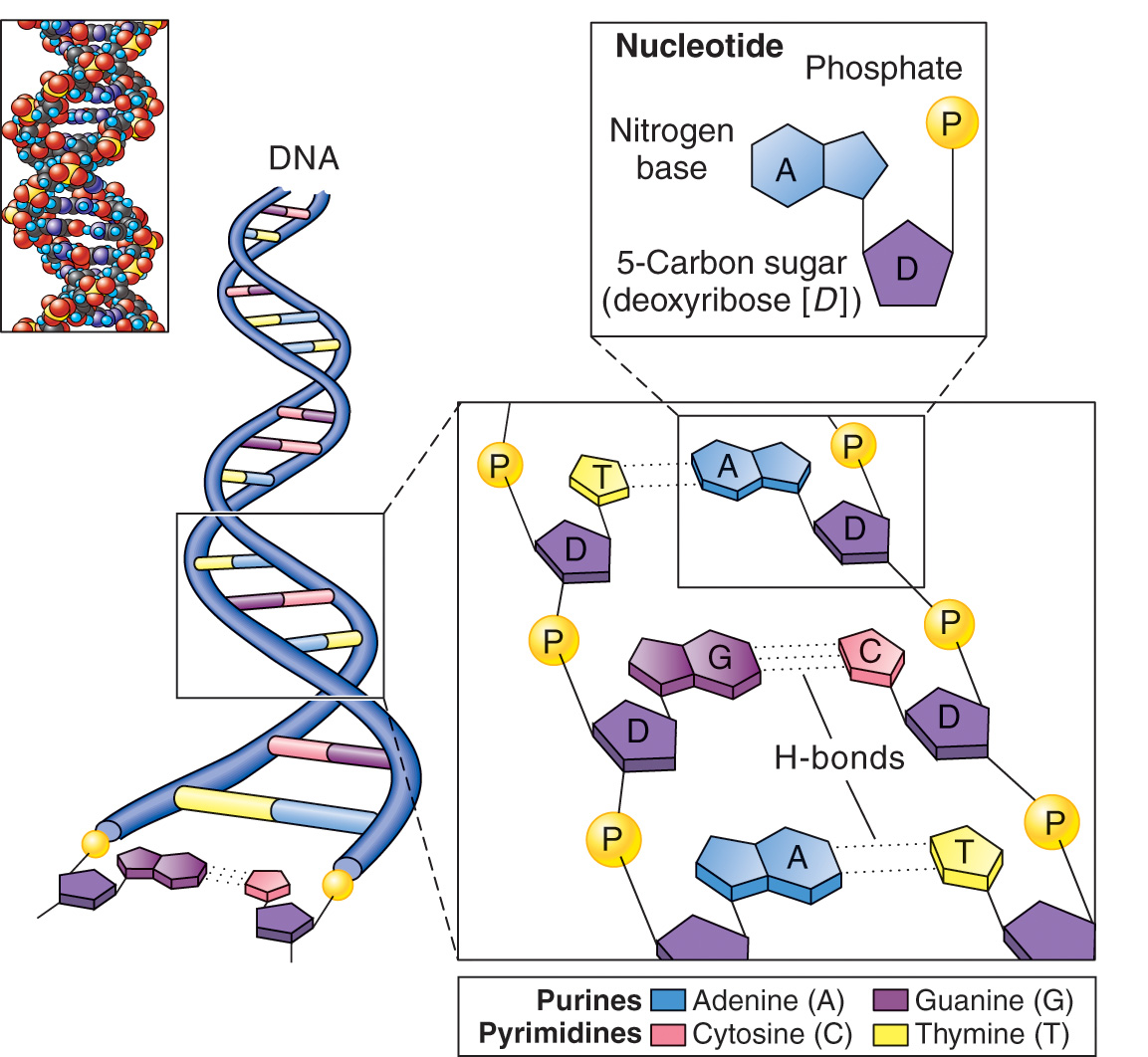
Purine bases — adenine and guanine; they have double ring structure.
Pyrimidine Bases — cytosine and thymine; they have a single ring structure.
Uracil replaces thymine in RNA.
DNA Molecules — the largest molecules in the body, are very large polymers composed of many nucleotides.
The nucleotides are joined together, saccharide group to phosphate group, by dehydration synthesis (condensation) to form a long sugar-phosphate backbone.
Two of these long polynucleotide chains compose a single DNA molecule. The chains coil around each other to form a double helix.
Each helical chain in a DNA molecule has its phosphate-sugar backbone toward the outside and its bases pointing inward toward the bases of the other chian.
Each base in a chain is joined to a base in the other chain by means of either two or three hydrogen bonds to form a base pair.
The two polynucleotide chains of a DNA molecule are thus held together by hydrogen bonds between the two members of each base pair.
RNA Molecules — consists of a single strand, it folds on itself to form a compact folded structure.
Each RNA strand is a sequence of ribonucleotides that is essentially copied from a portion of a DNA molecule.
It act as temporary copies of the master code of hereditary information in the DNA.
Types of RNA
RNA | DESCRIPTION |
Messenger RNA (mRNA) | Acts as a temporary copy of a gene from DNA. It carries the genetic code from the DNA in the nucleus to the ribosomes in the cytoplasm, where the proteins are synthesized. |
Transfer RNA (tRNA) | Responsible for bringing the correct amino acid to the ribosome during protein synthesis. It has an anticodon that matches with the mRNA codon, ensuring the correct sequence of amino acids in a protein. |
Ribosomal RNA (rRNA) | Forms the structural and functional components of ribosomes, which are the cellular structures where protein synthesis occurs. rRNA helps catalyze the assembly of amino acids into protein chains |
Small Nuclear RNA (snRNA) | Involved in the processing of pre-mRNA, including the removal of introns (non-coding regions) and splicing of exons (coding regions) to form mature mRNA |
MicroRNA (miRNA) | Small RNA molecules that regulate gene expression by binding to complementary mRNA sequences, leading to mRNA degradation or inhibition of translation. |
Long Non-Coding RNA (lncRNA) | Involved in regulating gene expression at various levels, including chromatin modification, transcription, and post-transcriptional processing. lncRNAs do not code for proteins but have roles in regulatory processes |
Ribozymes | RNA molecules that act as enzymes to catalyze biochemical reactions, such as the cleavage of RNA molecules during RNA processing. |
Nucleotides and related molecules
Adenosine Triphosphate (ATP) — a molecule composed of adenosine (adenine and ribose sugar), to which attached a string of three phosphate groups.
It is also called the energy currency of cells as it can pick up energy and give it to another chemical processes.
High energy bonds — covalent bonds that link the phosphate groups; because when they are broken during catabolic chemical reactions, the energy is released to form new compounds.
Squiggle lines in the structure of ATP.

Adenosine Diphosphate (ADP) — an inorganic phosphate group, a set of enzyme reactions splitted when they releases the energy that is stored in ATP.
Adenosine Monophosphate (AMP) — an inorganic phosphate group, the splitting of ADP.
Cyclic Adenosine Monophosphate — a one-phosphate molecule that is used as an intracellular signal within cells.
Creatine phosphate (CP) — a high-energy molecule made up of an amino acid derivative and a phosphate connected with a high-energy bond.
This is where muscles turn into, when ATP is short in energy supply.
When CP releases its phosphate group, the energy can be used to add a phosphate to ADP, thus “recharging” ATP.
A cell at rest has a relatively high ATP concentration, whereas an active cell has less ATP but is constantly rebuilding its stores.
An exhausted cell has a high ADP concentration and very low levels of ATP. It must resynthesize needed ATP to sustain its activity over time.
Cells at rest can recycle ADP and ATP and then reverse the cycle, thus reusing small amounts of ATP on a continuing basis.
Nicotinamide adenine dinucleotide (NAD+) and Flavin adenine dinucleotide (FAD) — act as coenzymes to shuttle energy-carrying particles (electrons) from one metabolic pathway to another during the many complicated steps of transferring energy from food molecules to ATP.

Combined Forms
Examples of Important Biomolecules
MACROMOLECULE | SUBUNIT | FUNCTION | EXAMPLE |
CARBOHYDRATES | |||
Glucose | Simple sugar (hexose: C 6 H 12 O 6 ) | Stores energy | Blood glucose |
Ribose | Simple sugar (pentose: C 5 H 10 O 5 ) | Plays role in expression of hereditary information | Component of RNA |
Deoxyribose | Simple sugar (pentose: C 5 H 10 O 4 ) | Plays role in storage and transmission of hereditary information | Component of DNA |
Glycogen | Glucose | Stores energy | Liver glycogen |
LIPIDS | |||
Triglycerides | Glycerol + 3 fatty acids | Store energy | Body fat |
Phospholipids | Glycerol + phosphate + 2 fatty acids | Make up cell membranes | Plasma membrane of cell |
Steroids | Steroid nucleus (4-carbon ring) | Make up cell membranes | Cholesterol, various steroid hormones Oestrogen |
Prostaglandins | 20-carbon unsaturated fatty acid containing 5-carbon ring | Regulate hormone action; enhance immune system; affect inflammatory response | Prostaglandin E, prostaglandin A |
PROTEINS | |||
Functional proteins | Amino acids | Regulate chemical reactions | Haemoglobin, antibodies, enzymes |
Structural proteins | Amino acids | Component of body support tissues | Muscle filaments, tendons, ligaments |
NUCLEIC ACIDS | |||
DNA | Nucleotides (sugar, phosphate, base) | Encodes hereditary information | Chromatin, chromosomes |
RNA | Nucleotides (sugar, phosphate, base) | Helps decode hereditary information; acts as “RNA enzyme”; silencing of gene expression | Transfer RNA (tRNA), messenger RNA (mRNA), double-strand RNA (dsRNA) |
NUCLEOTIDES AND OTHER RELATED MOLECULES | |||
Adenosine triphosphate (ATP) | Phosphorylated nucleotide (adenine + ribose + 3 phosphates) | Transfers energy from fuel molecules to working molecules | ATP present in every cell of the body |
Creatine phosphate (CP) | Amino acid derivative + phosphate | Transfers energy from fuel to ATP | CP present in muscle fibre as “backup” to ATP |
Nicotinic adenine dinucleotide (NAD + ) | Combination of two ribonucleotides | Acts as coenzyme to transfer high-energy particles from one chemical process to another | NAD + present in every cell of the body |
COMBINED OR ALTERED FORMS | |||
Glycoproteins | Large proteins with small carbohydrate groups attached | Similar to functional proteins | Some hormones, antibodies, enzymes, cell membrane components |
Proteoglycans | Large polysaccharides with small polypeptide chains attached | Lubrication; increase thickness of fluid | Component of mucous fluid and many tissue fluids in the body |
Lipoproteins | Protein complex containing lipid groups | Transport lipids in the blood | LDLs (low-density lipoproteins); HDLs (high-density lipoproteins) |
Glycolipids | Lipid molecule with attached carbohydrate group | Component of cell membranes | Component of membranes of nerve cells |
Ribonucleoprotein | Combination of RNA nucleotide and protein | Enzyme-like actions such as splicing mRNA | Small nuclear ribonucleoproteins (snRNPs or “snurps”) that make up the spliceosome structure in a cell |
Biomolecules and Disease
Biomolecule | Disease/Disorder | Description |
Carbohydrates | Diabetes Mellitus (DM) | A chronic disorder where the body cannot efficiently use glucose for energy due to impaired glucose transport into cells. |
Lipids | Hypercholesterolaemia / Hypertriglyceridaemia (Forms of Hyperlipidaemia) | High blood concentrations of cholesterol or triglycerides, often linked to heart disease and stroke. |
Proteins | Misfolded Protein Diseases (e.g., Phenylketonuria, PKU) | Diseases caused by protein misfolding or structural errors, such as missing or substituted amino acids. |
Nucleic Acids | Genetic Disorders | Diseases resulting from errors in the genetic code (mutations), which can lead to a wide range of disorders. |