Chemistry as a Foundation for Biology
0.0(0)
Card Sorting
1/207
There's no tags or description
Looks like no tags are added yet.
Last updated 2:38 AM on 9/14/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
208 Terms
1
New cards
How do we define elements?
specific number of protons and neutrons
2
New cards
How do you know the number of electrons?
same as protons
3
New cards
Why are electrons important?
means by which atoms interact and bond, creating molecules which biologists care a lot about, critical in thinking about energy
4
New cards
What do elements in a column (group) have in common?
same number of valence electrons
5
New cards
Valence electrons
electrons on the outermost orbital
6
New cards
What do elements in a row (period) have in common?
same number of electron orbitals
7
New cards
Elements of life
carbon, oxygen, hydrogen, nitrogen, phosphorus, sulfur, potassium, calcium, sodium, chlorine, magnesium
8
New cards
Chemical bond
a ‘stable’ attraction between two atoms
9
New cards
How are the number of bonds an atom can form determined?
number of unpaired (valence) electrons in the outermost shell
10
New cards
HONC1234
Hydrogen 1 bond, oxygen 2 bonds, nitrogen 3 bonds, carbon 4 bonds
11
New cards
How is the nature of a bond determined?
relative electronegativities of two atoms
12
New cards
electronegativity
a measure of an elements’ ability to attract electrons
13
New cards
How can you determine electronegativity from the periodic table
going up and to the right is more electronegative
14
New cards
What is the relationship between atomic radius and electronegativity?
the higher the atomic radius, the lower the electronegativity
15
New cards
Why does the inverse relationship between atomic radius and electronegativity exist?
electron orbital is closer to the nucleus in smaller atoms
16
New cards
What type of bonds do atoms that have similar electronegativities form?
equal sharing of electrons
17
New cards
What kind of bonds do atoms with very different electronegativities form?
transfer of electrons
18
New cards
Nonpolar covalent bonds
similar electronegativities, atoms have no charge
19
New cards
Polar covalent bonds
slightly different electronegativities, atoms have a partial charge
20
New cards
Ionic bonds
very different electronegativities, atoms have a full charge because an electron was “stolen”
21
New cards
Why does the addition of a phosphate group have an effect on the structure and function of the molecule?
the oxygens make their end of the molecule more electronegative meaning that it interacts more with polar molecules
22
New cards
Explain the role relative electronegativity plays in bond formation.
relative electronegativity, or the electronegativity that bonded atoms have in relation to each other, plays a large role in bond formation because when two atoms have vastly different electronegativities, they will form an ionic bond, whereas atoms with some difference in electronegativity will form polar covalent bonds, and atoms with very little or no difference will form nonpolar covalent bonds.
23
New cards
What is the guiding “mantra” of biology?
structure \= function
24
New cards
When talking about solutions, what is water referred to as?
universal solvent
25
New cards
Important functional groups
hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, sulfhydryl
26
New cards
Organic molecules
proteins, carbohydrates, nucleic acids, lipids
27
New cards
Solvent
what you dissolve something into
28
New cards
Why does water have partial charges?
polar covalent bonds within them due to differing electronegativity
29
New cards
Hydrogen bonds
partial charges cause attraction between oxygen of one molecule and hydrogen of another, relatively weak in terms of bonding which allows liquid water to change shape
30
New cards
What happens to hydrogen bonds when energy is added to the system?
hydrogen bonds are broken (boiling and turning to gas)
31
New cards
What happens to hydrogen bonds when energy is added to the system?
hydrogen bonds become stronger (ice formation)
32
New cards
Cohesion
water molecules stick together
33
New cards
Adhesion
water molecules sticking to other surfaces
34
New cards
What kinds of molecules does water “like” to stick to?
polar molecules
35
New cards
Surface tension
when cohesion is greater than adhesion
36
New cards
Specific heat
energy needed to raise temperature
37
New cards
What properties of carbon make it a logical backbone for organic molecules?
4 valence electrons allows 4 bonds to occur, tetrahedral shape of bonding, optimal size for electronegativity
38
New cards
If so many molecules have carbon, why are they different?
different structure and other molecules involved
39
New cards
R group
stands in for “something else”
40
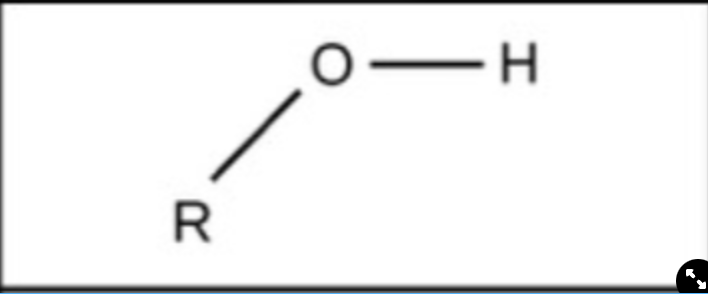
New cards
Hydroxyl
polar
41
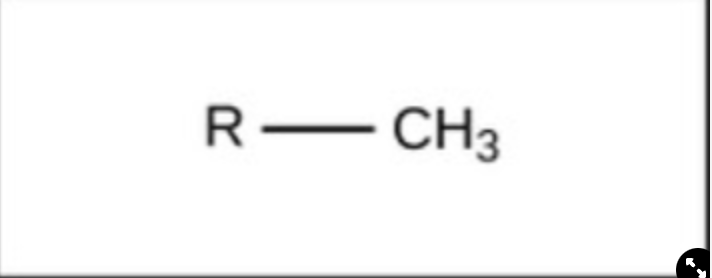
New cards
Methyl
nonpolar
42
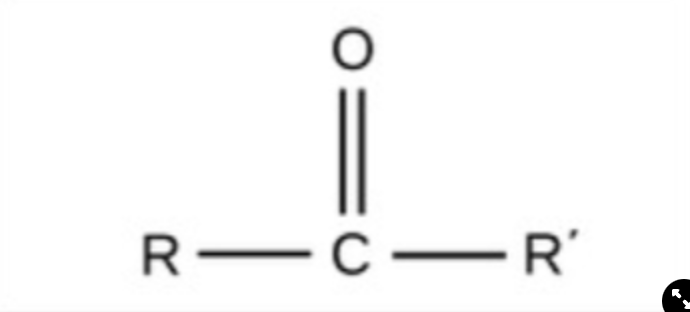
New cards
Carbonyl
polar
43
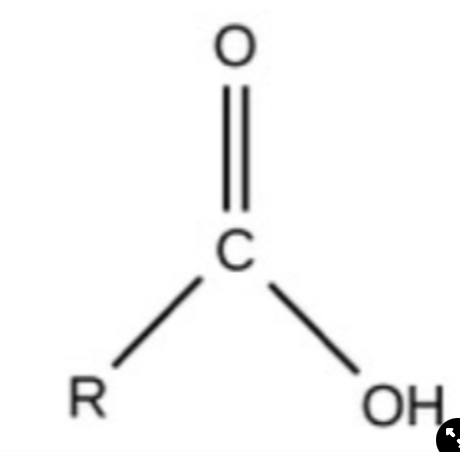
New cards
Carboxyl
charged, ionizes to release H+, acidic
44
New cards
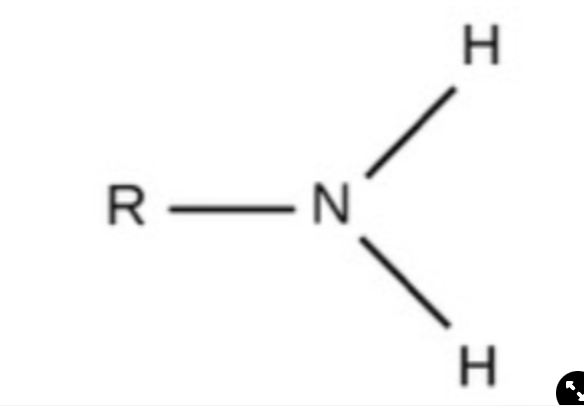
Amino
charged, accepts H+ to form NH3+, basic
45
New cards
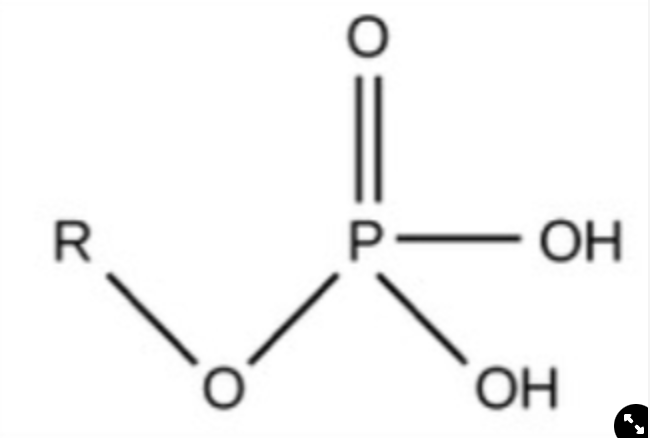
Phosphate
Charged, ionizes to release H+, acidic
46
New cards
Sulfhydryl
polar
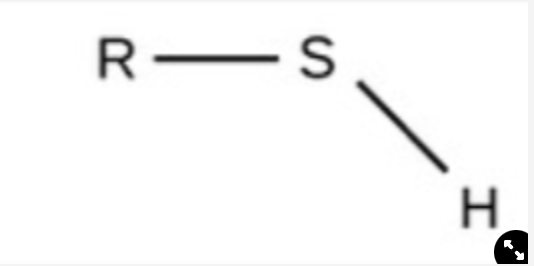
47
New cards
What are organic molecules made up of?
part hydrocarbons (hydrogens and carbons) and part functional groups which typically include oxygen, nitrogen, phosphorus, and sulfur
48
New cards
Hydrophilicity
polar molecules and ions dissolve readily in water
49
New cards
Hydrophobicity
nonpolar molecules do not dissolve readily in water
50
New cards
Amphipathic
a molecule that has both polar and nonpolar properties
51
New cards
What is the structure/function relationship of water that allows it to play such a central role in biology?
the structure with oxygen being more electronegative than hydrogen causes polarity allowing it to interact more specifically with charged and partially charged molecules
52
New cards
Why do hydrophilic and hydrophobic mean “loved by water” and “hated by water” rather than “loving water” and “hating water”?
water is the focus of the word and the concept
53
New cards
What do we mean by “dissolve” chemically?
atoms split from their molecular partners
54
New cards
Function of proteins
“workers” of life, 55% of cell mass
55
New cards
Structure of proteins
made of amino acids, shapes driven by functional groups
56
New cards
Function of carbohydrates
energy, structural integrity, 10% of cell mass
57
New cards
Structure of carbohydrates
made of sugars, long chains, hydrophilic
58
New cards
Function of nucleic acids
cell energetics, information flow, 25% of cell mass
59
New cards
Structure of nucleic acids
made of nucleotides, mostly hydrophilic, helical in shape
60
New cards
Function of lipids
cell energetics, structure, signaling, 10% of cell mass
61
New cards
Structure of lipids
made of fatty acids, three types, mostly hydrophobic
62
New cards
3 types of lipids
fats, phospholipids, steroids
63
New cards
Why do oil and water not mix?
oil is nonpolar and water is polar which is why they cannot interact with each other
64
New cards
How does salt melt ice?
the salt interferes with the bonds between the water, causing it to change state from solid to liquid
65
New cards
How does guar gum reduce the abundance of ice crystals in ice cream?
guar gum is hydrophilic so it interrupts the bonds between the water
66
New cards
Structure of amino acids
wide range
67
New cards
Function of amino acids
enzymes, signals, structural
68
New cards
Structure of carbohydrates
regular, repeating structures
69
New cards
Function of carbohydrates
cell walls, energy
70
New cards
Structure of nucleic acids
helical
71
New cards
Function of nucleic acids
information
72
New cards
Structure of lipids
hydrophobic or amphipathic
73
New cards
Function of lipids
membranes, energy, signals
74
New cards
Chemical reactions
changes to the sharing of electrons and the rearrangement of bonds
75
New cards
Catabolic reaction
breaking down molecules into subunits/monomers
76
New cards
Anabolic reaction
building molecules into macromolecules/polymers
77
New cards
How to remember CATabolic reaction
cats knock things off counters which makes them break
78
New cards
Hydrolysis reaction
breaking down molecules requires water addition
79
New cards
Dehydration reactions
building molecules removes water from the organic molecule
80
New cards
Formation of proteins
amino acid + amino acid \= protein + water; anabolic and dehydration
81
New cards
ATP hydrolysis
ATP + H2O \= ADP + Pi; catabolic, hydrolysis
82
New cards
Gibbs Free Energy Equation
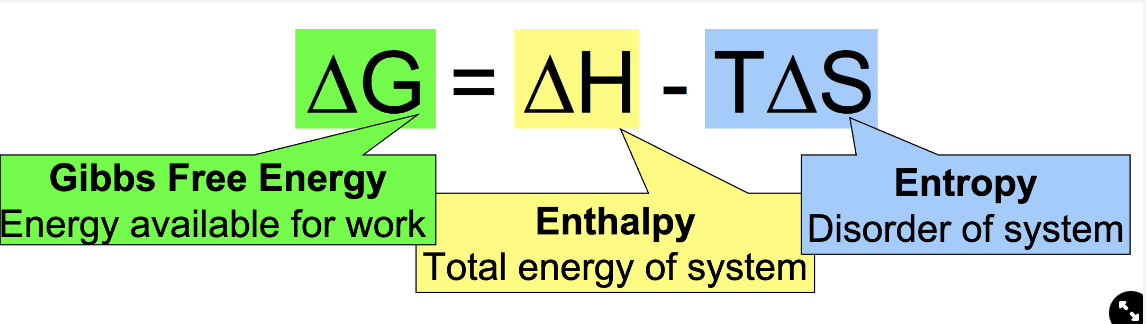
83
New cards
Gibbs free energy
energy available for work
84
New cards
Enthalpy
total energy of the system
85
New cards
1st law of thermodynamics
enthalpy
86
New cards
Why do the electrons in the outermost shells have the greatest energy?
requires potential energy to hold the negatively charged electrons away from the positively charged nucleus
87
New cards
For anabolic reactions, is the change in enthalpy positive or negative?
positive
88
New cards
For catabolic reactions, is the change in enthalpy positive or negative?
negative
89
New cards
Entropy
disorder of system
90
New cards
2nd law of thermodynamics
entropy
91
New cards
For an anabolic reaction, is the change in entropy positive or negative?
negative
92
New cards
For a catabolic reaction, is the change in entropy positive or negative?
positive
93
New cards
Endergonic
absorbs of energy
94
New cards
Exergonic
releases of energy
95
New cards
What determines protein shape?
amino acid sequences and the environment
96
New cards
What determines protein function?
protein shape
97
New cards
How do amino acids have diverse chemical identities?
different R groups
98
New cards
Types of proteins
antibodies and complement, contractile and motor, enzymes, hormones, receptors, structural, transport
99
New cards
Amylase
breaks down starch into sugars
100
New cards
Histone deacetylase
determines which genes to express