Brewer Fluid, Electrolyte Acid-Base
1/113
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
114 Terms
What is the focus of this chapter?
Homeostatic mechanisms that regulate body fluids and are critical to cells.
How much fluid makes up total body composition? (percentage)
50-60%
What are minerals?
Inorganic substances that are dissolved within and form ions called electrolytes
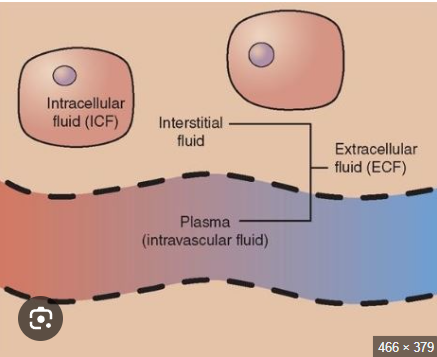
Name the two components of fluid compartments
Intracellular fluid (ICF)
Extracellular fluid (ECF)
Describe intracellular fluid
fluid inside a membrane
water content varies most here
Why does water content vary in ICF?
This is because of variation in tissue types (muscle vs fat)
Why is ICF distinct with extracellular fluid? (ECF)
Due to plasma membrane transport
Describe ECF
fluid not inside a membrane
interstitial volume varies
How does the volume of blood vary between the sexes
Men have a higher volume of blood than women
Here’s a picture to help visualize ICF & ECF
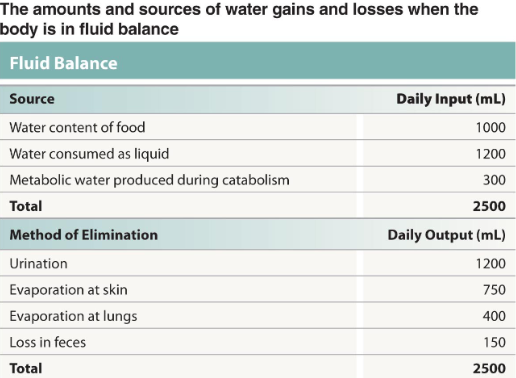
What is meant by the term “fluid balance”
water content stable over time
think in terms of gains and losses
How do we gain fluids in our body?
Primarily by absorption along GI tract
How do we specifically gain water?
As nutrients and ions are absorbed, an osmotic gradient is created causing passive absorption of water
How do we lose the fluids in our body?
Mainly through urination, but other routes as well
digestive secretions are reabsorbed similarly to ingested fluids
What percentage of urine accounts for the fluid being loss?
over 50%
Give an overview of what occurs when the body maintains fluid balance (in terms of gains and losses)
The source and method of elimination of water are equal
Describe what occurs with ECF and ICF when they are balanced
Although different compositions, they’re at osmotic equilibrium
How is loss of water from the ECF replaced?
replaced with water in ICF
Describe a fluid shift
occurs in minutes to hours and restores osmotic equilibrium
What happens when to the ECF and ICF when a person is dehydrated
results in long term transfer that cannot replace ECF water loss
homeostatic mechanism to increase ECF fluid volume will be employed
NOW LET’S STUDY MINERAL BALANCE
Describe mineral balance
equilibrium between ion absorption and excretion
Where does major ion absorption occur?
Small intestine
colon
Where does major ion excretion occur?
Kidneys
Which glands excrete ions and water variably
sweat glands
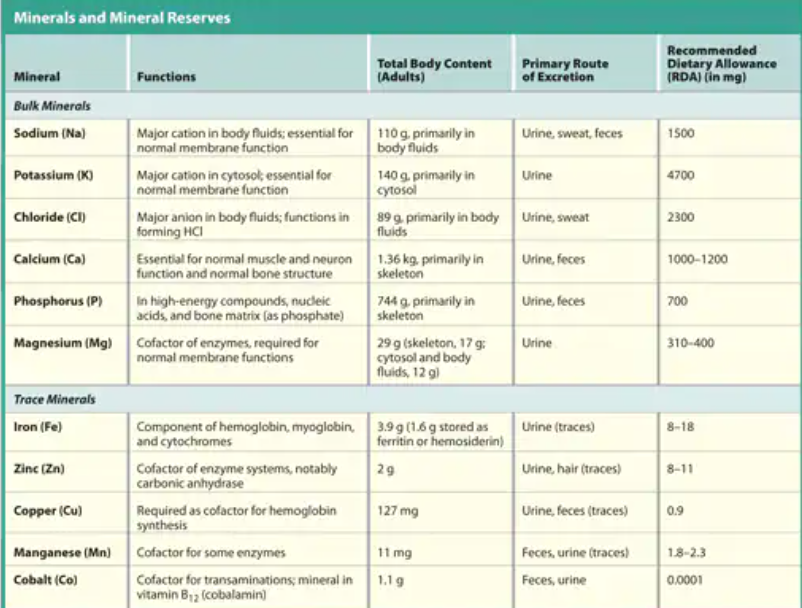
Where does ion reserve mainly occur?
Primarily in the skeleton (bone)
Which two ions are not stored in the bone?
Sodium and Potassium are not stored in the bone
remains in solution
Describe the overall pathway food and minerals take in the body
Digestive
ECF
ICF
Bone
Excreted through sweat and kidneys
Ion absorption occurs in the ___________ while ion excretion occurs at the _______
GI tract
kidneys
What are the mechanisms in which sodium is absorbed/move through solution?
channel mediated diffusion
co transport
active transport
Which electrolytes are absorbed through active transport (Hint* Sam & Cat make idiot people suffer)
Sodium (can be)
Calcium
Magnesium
Iron
Phosphate
Sulfate
Which electrolytes are important for normal membrane function
Sodium and potassium
Here’s a chart for some major functions of electrolytes
Moving on to WATER AND SODIUM BALANCE 🙂
Describe sodium balance
when sodium gains equal losses
Which type of fluid responds to changes in sodium
Relatively small changes in sodium are accommodated by changes in ECF volume
Homeostatic responses involve what two parts?
ADH control of water loss/retention by kidneys and thirst
Fluid exchange between ECF and ICF
When sodium gains exceed losses, the ECF volume __________
INCREASES
When sodium losses exceed gains, the volume of the ECF _____
DECREASES
Describe the overall process of restoring homeostasis when sodium levels in the plasma increase
1) Osmoreceptors in hypothalamus are stimulated
2) ADH secretion increases (restricts water loss and stimulates thirst)
3) Because ECF osmolarity increases, water shifts out of the ICF, increasing ECF volume and lowering ECF Na+ concentrations
4) Homeostasis restored- decreased levels of sodium in the ECF (which leads to normal)
Describe the overall process of restoring homeostasis when sodium levels in plasma decrease
1) Osmoreceptors in hypothalamus inhibited
2) ADH secretion decreases (thirst is suppressed and water losses at the kidneys increase)
3) water loss reduces ECF volume, concentrates ions
4) Homeostasis restored-increased sodium levels
If ECF volume increases, blood volume ________
increases
Exchange in sodium are accommodated by changes in…
Blood pressure
Blood volume
Describe hyponatremia
Not enough sodium in the blood
low ECF sodium concentration
Hyponatremia is low sodium concentration of <__________ mEq/L
136 mEq/L
Why could hyponatremia occur?
can occur from overhydration or inadequate salt intake
Describe hypernatremia
high ECF sodium concentration
Hypernatremia is high sodium concentration of >__________ mEq/L
145 mEq/L
What could be a cause from hypernatremia
Commonly from dehydration
Sodium changes are accompanied by change in blood pressure and volume, knowing this, describe the mechanisms that occur when blood pressure and volume INCREASE
1) Natriuretic peptides released by cardiac muscle cells
2) This causes increased sodium and water loss in urine and reduced thirst
3) Inhibition of ADH, aldosterone, epinephrine, and norepinephrine release
4) These combined effects cause reduced blood volume and pressure
5) Homeostasis restored-ECF volume decreases
Sodium changes are accompanied by change in blood pressure and volume, knowing this, describe the mechanisms that occur when blood pressure and volume DECREASE
1) Increased renin secretion and angiotensin II activation, increased aldosterone release, and increased ADH release
2) Causes increased urinary sodium retention, decreased urinary water loss, increased thirst, and increased water intake
3) Homeostasis restored-ECF volume increases
Let’s move on to POTASSIUM IMBALANCE
Describe potassium balance
When potassium gain equals potassium loss
How does major gain of potassium occur?
Major gain is through the digestive tract absorption
How much potassium is gained per day through the GI tract?
Approximately 100mEq (1-9-5.8 g)/day
How is potassium lost?
Major loss is excretion by the kidneys
Describe potassium loss by the kidneys
Controlled by aldosterone regulating the sodium and potassium exchange pumps in the DCT and collecting duct of the nephron
What happens when the ECF pH gets low?
Can cause H+ to be substituted for K+
Is potassium highest in the ECF or ICF?
ICF due to Na+/K+ exchange pump
Compare the numbers of potassium in the ICF vs ECF
135 mEq/L in ICF vs 5 mEq/L in ECF
What two factors affect potassium balance?
The rate of potassium entry across the digestive epithelium and the rate of potassium loss into the urine
What percent of potassium is in the ICF compared to the ECF
98%
When the blood concentration of potassium falls below 2 mEq/L, this is referred to as…
Hypokalemia
What 2 things are hypokalemia caused by?
Diuretics
Aldosteronism
What is aldosteronism?
Excessive aldosterone secretion
aldosterone is a steroid hormone produced by the adrenal glands which plays a role in regulating blood pressure and electrolyte balance
What are the symptoms of hypokalemia
muscular weakness, followed by paralysis (potentially lethal when affecting the heart)
When the blood concentration of potassium rises above 8 mEq/L, this is referred to as…
Hyperkalemia
Hyperkalemia is caused by
chronically low pH
Kidney failure
drugs promoting diuresis by blocking Na+/K+
What are the symptoms of hyperkalemia?
Muscular spasm including heart arrythmias
SECTION 2: ACID BASE BALANCE
Describe acid base balance
when H+ production equals loss
What’s normal plasma pH?
7.35-745
Many _______ activities produce acids
metabolic
What are some metabolic activities that produce acids?
Carbon dioxide —> carbonic acid from aerobic respiration
Lactic acid from glycolysis
How is carbon dioxide eliminated?
Through the respiratory system
How is H+ lost and stored?
Lost by H+ excretion from kidneys
Buffers temporarily store H+
What are fixed acids?
Acids that do not leave solution and remain in body fluids until kidney secretion
Name some fixed acid examples and how they are generated
sulfuric and phosphoric acid
generated during catabolism of amino acids, phospholipids, and nucleic acids
What are organic acids?
Acids that are part of cellular metabolism
Name some examples of organic acids
lactic acid and ketones
Organic acids are metabolized _________ so no _____________
rapidly, accumulation
What are volatile acids?
Acids that can leave the body by external respiration
What’s an example of a volatile acid?
Carbonic acid (H2CO3)
Describe acidemia
when the pH of blood decreases below 7.35. The physiological state that results is called acidosis.
Acidosis is more common due to acid producing __________ activities
metabolic
What are the effects of acidosis?
CNS function deteriorates, may cause coma
cardiac function contractions grow weak and irregular
peripheral vasodilation causes BP to drop
Describe alkalemia
When the pH of blood increases above 7.45. The physiological state that results is called alkalosis
can be dangerous but relatively rare
What’s an important factor affecting body pH and why?
The partial pressure of CO2
because carbon dioxide combines with water to form carbonic acid; this is a reversible reaction that can buffer body pH
adjustments in respiratory rate can affect body pH
Partial pressure of CO2 and pH have a ____________ relationship
inverse
Describe the inverse relationship between partial pressure of CO2 and pH
When CO2 levels rise, more carbonic acid forms, additional hydrogen ions and bicarbonate ions are released, and the pH goes down.
Describe the function of a buffer
a substance that tends to oppose changes in the pH of a solution by removing or replacing hydrogen ions
Buffers maintain blood pH within normal limits (7.35-7.45)
A buffer system typically consists of…
a weak acid (HY) and the anion (Y-)
What does adding H+ to a buffer solution do?
Results in the formation of additional molecules of the weak acid
What does removing H+ from a buffer solution do?
results in the dissociation of additional molecules of HY. This releases H+
Describe one limit of a buffer system
They can only temporarily affect pH (H+ not eliminated)
Name the three buffer systems
Phosphate buffer system
carbonic acid-bicarbonate buffer system
Protein buffer system
Describe the function of the phosphate buffer system
buffers pH of ICF and urine
Describe the carbonic acid-bicarbonate buffer system
most important in the ECF
fully reversible
bicarbonate reserves contribute (from NaHCO3 in ECF)
Describe the protein buffer system
contribute to the regulation of pH in the ECF and ICF
usually operate under acidic conditions
binding to carboxylic and amino group
Name 3 examples of a protein buffer system
hemoglobin buffer system
amino acid buffers (all proteins)
plasma protein buffers