chemistry mixtures and resources revision
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
diffusion
Movement of molecules from an area of higher concentration to an area of lower concentration down the concentration gradient.
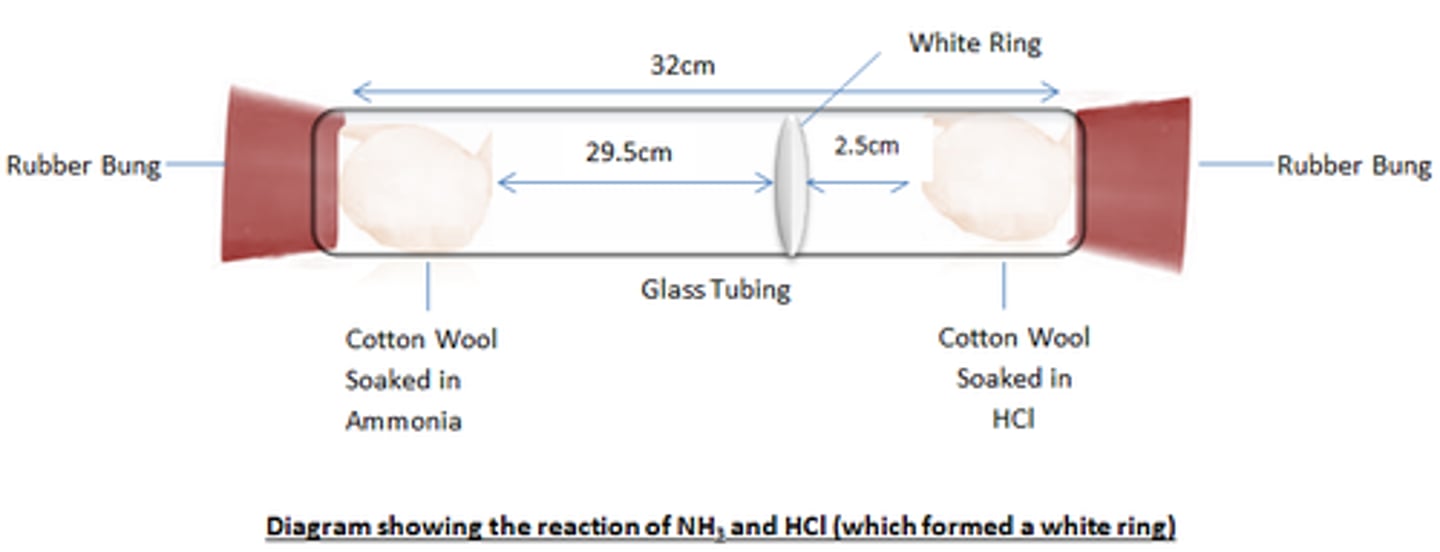
NH3 and HCl Experiment
in a tube with two ends, take two pieces of cotton wool soaked in HCl and NH3 and insert into either end of the tube, sealing with a bung.
The reaction of the two gases gives ammonium chloride
NH3 + HCl -> NH4Cl
both molecules have to diffuse through the air in the tube colliding with air particles, until they meet and react forming ammonium chloride which is a white ring
because ammonia is lighter (lower molecular mass than HCl, the ring is closer to the HCl) as the NH3 covers more distance in the same time
Factors that affect the rate of diffusion (rate is how much in a given time)
molecular mass - the lighter the molecule, the faster it can move and therefore diffuse faster
concentration gradient - the larger difference in concs. of particles in two given areas : from the area the substance travels from and the area it travels to the higher diff rate
e.g. water diffusing from a dilute salt solution to a concentrated salt solution
temp - higher temp, more energy in the Kin-str of particles to diffuse faster
chromatography
used to separate mixtures (of colours) of substances with specific solubilities
take chromatography paper and draw a pencil line 2 cm above the edge of the paper, then add ink spots on the paper on the pencil line,
then in a beaker with a small amount of solvent, place the paper in a beaker of water with the edge barely touching the solvent
how it works
The water is drawn up the paper by capillary action (adhesive force between the paper and water because of hydrogen bonding),
the water travelling up the paper is called the mobile phase
When the solvent reaches the ink spots (analyte) they are dissolved and carried with the water as it moves up the paper
the ink on the paper is called the stationary phase
the inks (analyte) contain a mixture of inks which have different attractions and solubilities so some dissolve and move up further than others - therefore inks with the same colour and distance travelled can be the same
factors that affect how far the analytes travel
solubility = attraction to the mobile phase - the more soluble the ink, the further it travels
the more attracted (affinity) the ink has to the stationary phase, the less distance travelled, as it has less attraction to move up with the water
Strong attraction to stationary phase = less movement (more "stuck")
Weak attraction to stationary phase = more movement (more "mobile" with the solvent).
why the base line is drawn in pencil (+ other errors)
so the solvent cannot dissolve the pencil and move up the paper with it, disrupting the mobile phase
if the line was drawn in pen, then the ink would dissolve in the solvent and move up the paper, disrupting mobile phase
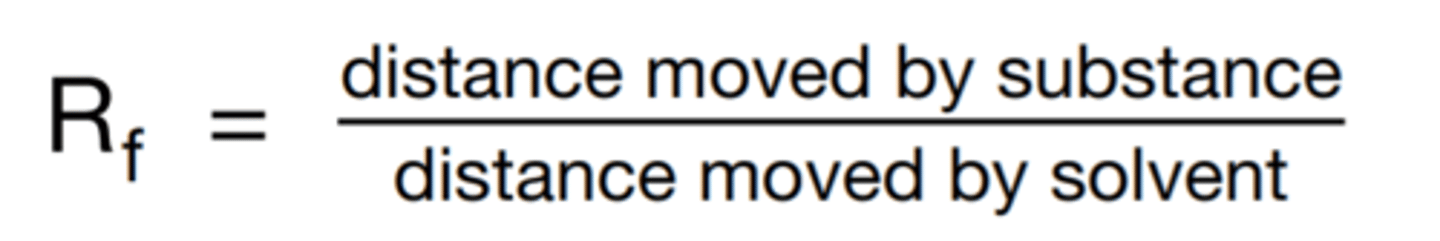
calculating Rf values
make sure the units are the same (dot over snot) !
key words for distillation
solvent : the substance that dissolves the solute
solute : the substance that dissolves in the solvent
dissolve : disperse a solute at the molecular or ionic level within a solvent, forming a homogeneous solution
miscible : forming a homogenous mixture
immiscible : forming a heterogenous mixture e.g. oil and water
dissolving is homogenous because the molecules evenly disperse
distillation
because different substances in a mixture have different boiling points, evaporate one liquid by heating of the lower BPT then condense the vapour back into a liquid by cooling
flask is connected to a long continuous tube with a thermometer at the top and the other part of the tube having a condenser jacket, circulating cold water around the tube
as the liquid evaporates the thermometer reading increases
fractional distillation - mixture of diff. liquids, however they must have diff. boiling points, and they can have closer boiling points
in the continuous tube, there is a fractionating column filled with lots of glass beads, other then that same setup as simple distill.
gently heat mixture, both will start to evaporate, but the lower bpt will start to evap more easily - mixture of two diff mixtures
once they reach the fractionating column they condense where the liquids evaporates again, this repeated evap and conden increases the amount of the lower bpt liquid passing through the f.column
temp readings for fractional distillation
the vapours reach the t.meter and the temp begins to rise, when the temperature is rising a mixture of two diff vapours are passing over the t.meter, however this will be relatively more of the lower bpt mixture
when the temp is constant, the lower bp mixture is evaporating and this can be collected in a fresh beaker,
once the temp starts to increase again, more of the higher bpt is passing over, then condenses, second fraction
liquids with closer bpts are much harder to separate
pure substance
can be a single element or compound -a pure substance is a single element or compound, not mixed with any other substance.
they have specific bpts and specific fixed mpts, e.g. with water it should melt @ 0c and not have a range for melting and/or boiling, but impure water has ranges of where it melts and boils
formulation
A complex mixture that has been designed as a useful product
quantity of each component is carefully measured so that the product has the properties we need
e.g. food, fuel, cleaning products, medicine
potable water
water safe to drink - needs to have sufficiently low levels of dissolved substances (e.g. salts) and very low levels of microbes
potable ≠ pure - as pure contains no dissolved substances at all, it is just water with no salts of microbes with pH 7- potable water contains salts and microbes in very small quantities and pH may also not be 7
sources of potable water
in UK, rain water provides most potable water, because it contains low levels of dissolved substances
rain water collects in fresh water places like rivers, aquifers, underground streams, lakes etc.
1. for potable water choose a good source of fresh water
potable water after choosing a source
sedimentaion (coagulation) occurs where the water is given time for all the suspended particles to settle, or a coagulant like aluminium sulfate reacts with the suspended particles causing them to clump together (flocculation) and sink
pass the water through filter beds to remove suspended particles (flocks) and materials like leaves
then water is sterilised to kill microbes, UK chlorine is used however ozone (O3) or UV light can be used
potable water with the scarcity of freshwater
Fresh water is abundant in UK and has very low levels of dissolved minerals, however freshwater is scarce in many places and only way to get potable water is by sea water which has very high levels of dissolved minerals
in this case potable water is produced by desalination which reduces the levels of dissolved minerals down to be acceptable as potable
one way of desalination can be distillation
reverse osmosis : - water goes from a lower concentration through a semi permeable membrane
both RO and distill. require lots of energy and can be expensive but both reduce the amount of dissolved substances in the water
to check whether water is pure by pH
1. check pH, as pure water has pH 7 , few drops of sample on universal indicator paper, if not 7, there are dissolved acid or alkali therefore not pure
even if pH is 7, then the sample could also contain dissolved solids
to check for dissolved solids in water
record mass of empty evap. basin on a BALANCE, then fill bain with water and place this on a tripod on gauze heated over a bunsen, gently heat water until all has evaporated, allow it to cool, then weigh again
if the water contained any dissolved solids, then the mass of the empty basin will have increased, because h20 has dissolved but the dissolved solids would have formed crystals
therefore not pure
if did not increase then sample is pure, however the water still may contain dissolved gases
waste water
all of this waste water from e.g. hygiene or agriculture contains a very large amount of organic molecules e.g. from urine or faeces and pathogens like bacteria
therefore must by carefully treated before being released back into the environment
treating waste water (sludge)
sewage is screened by passing through a mesh to remove solids
sedimentation, either chemically, or settling - this produces a semi-solid sludge at the bottom from denser particles sinking and an effluent at the top (liquid)
effluent is taken away and the sludge is digested using anaerobic bacteria, anaerobically the bacteria produce biogas which can be used as fuel, and the digested sludge can be used as a fertiliser for farming
treating waste water (effluent)
liquid effluent contains large amounts of organic molecules and pathogens that need to be reduced
air is bubbled through the effluent which causes the aerobic bacteria to multiply. In the presence of O2 the aerobic bacteria digest the organic molecules and microbes (pathogens)
now the effluent can be safely discharged into the nearby rivers or sea,
or be sterilised to be potable
industrial processes
in industrial processes water can be polluted with harmful chemicals which need to be removed before the water can enter sewage treatment
comparing aquifers, salt water, and waste water in ease to produce potable water
easiest way is by aquifer ground water, usually safe to drink after sterilised with chlorine - but can sometimes be polluted with e.g. fertilisers from farms so needs to be tested
potable water can be made from waste water e.g. sewage, but requires multiple purification stages so lots of energy and less economical - so only in places where water is scarce
salt water needs to be desalinated but requires lots of energy and is expensive