AP Environmental Science - Unit 7: Atmospheric Pollution
7.1 Intro to Air Pollution
air pollution - introduction of chemicals, PM, or microorganisms into atm at concentrations high enough to harm plants, animals, and materials such as buildings, or to alter ecosystems
FRQ TIP! → write about air pollutants specifically, don’t just write “pollution”
air pollutants
clean air act identified 6 criteria air pollutants that the epa is required to set acceptable limits for, monitor, and enforce
SO2 → sulfur dioxide
coal combustion
NOx → nitrogen oxides (NO & NO2)
all FF combustion
CO → carbon monoxide
incomplete combustion
PM → particulate matter
FF/biomass combustion
O3 → ozone (tropospheric)
photochemical oxidation of NO2
Pb → lead
metal plants, waste incineration
greenhouse gases
CO2 is NOT an air pollutant according to the clean air act
not included on FRQ guides for air pollutants
does not directly lower air quality from a human health standpoint
not toxic to organisms to breathe
not damaging to lungs/eyes
does not lead to smog or decreased visibility
CO2 is a ghg; it does lead to earth warming → env and human health consequences
sources of pollution
combustion
factories/power plants (energy)
coal releases more air pollutants than other FFs
releases CO, CO2, SO2, NOx, toxic metals (mercury, arsenic, lead), and PM (often carries the toxic metals)
vehicles
natural resources (volcano, forest fire, lightning, plants, decaying matter, etc.)
primary air pollutants - polluting compounds that come directly out of a smokestack, exhaust pipe, or natural emission source
NOx, CO, CO2, VOCs, SO2, PM, hydrocarbons
secondary air pollutants - primary pollutants that have undergone transformation in the presence of sunlight, water, oxygen, or other compounds
tropospheric O3 (ozone)
sulfuric acid (H2SO4) and sulfate (SO4 2-)
nitric acid (HNO3) and nitrate (NO3-)
7.2 Photochemical Smog
photochemical smog
forms when NOx chemicals mix with VOCs
mix with heat and sunlight to produce smog and other secondary pollutants
NO2 (+ sunlight) → NO + O → O + O2 → O3 (ozone)
volatile organic compounds - chemicals that vaporize quickly (go from liquid to gas)
common VOCs → benzene, ethylene glycol, formaldehyde, gasoline, trees (natural)
other contributing factors
time of day → nitrogen oxides are produced in the morning from vehicle combustion
ozone tends to peak in the afternoon
season → summer is sunniest, so more reactions
location → urban areas
heat island effect → higher temps = faster evaporation of VOCs
more VOCs - gas stations, laundromats, factories
more NOx emissions - more vehicles, power plants
impacts of smog
env → reduces sunlight, limits photosynthesis; ozone damages plant stomata (opening to take in CO2) and animal respiratory tracts
human → respiratory irritant; worsens asthma; COPD; irritates eyes
reduction of smog
decreasing the number of vehicles on the road decreases NO2 emissions
fewer vehicles = less gas = fewer VOCs
carpooling, public transport, biking, walking, working from home
increased electricity production from renewable sources that don’t emit NOx (solar, wind, hydro)
natural gas power plants release far less NOx than coal
7.3 Thermal Inversion
thermal inversion
under normal conditions, where temperatures decrease with increasing altitude, emissions rise into the atmosphere
the warm layer of air trapped between the two cooler layers in known as an inversion layer
inversions occur because the ground cools quicker than the air above
due to this layer, convection doesn’t carry pollutants away
inversion layer traps smog, pm, and smoke
effects of thermal inversion
air pollutants trapped closer to earth (smog, PM, SO2, NOx)
respiratory irritation
env effects - decreased photosynthesis rate
econ effects - decreased tourism revenue
7.4 Atmospheric CO2 & PM
natural sources of air pollutants
lighting strikes
convert N2 in atm to NOx
plants
plants emit VOCs
ex) terpenes and ethylene from pine, fir, spruce trees
forms natural photochemical smog in smoky mnts
forest fires
CO, PM, NOx
combustion of biomass also releases CO2 and H2O vapor (GHGs)
volcanoes
SO2, PM, CO, NOx
natural sources of CO2 & PM
respiration
all living things release CO2 through respiration
aerobic decomposition
decomposition of organic matter by bacteria and decomposers in the presence of oxygen → releases CO2
natural PM sources
sea salt, pollen, ash from forest fires and volcano dust (windborne soil)
leads to haze (scattering of sunlight and reduced visibility
anaerobic decomposition
decomposition of organic matter by bacteria and decomposers in low or oxygen-free conditions → releases CH4
PM10 vs PM2.5
particulate matter - solid/liquid particles suspend in air (also referred to as “particulates”)
PM10 (<10 micrometers)
particles or droplets like dust, pollen, ash, or mold
too small to be filtered out by nose hairs and trachea cilia; can irritate respiratory tract and cause inflammation
PM2.5 (<2.5 micrometers)
particles from combustion (especially vehicles)
more likely to reveal deep into the lungs due to smaller size
associated with chronic bronchitis and increased risk of lung cancer
7.5 Indoor Air Pollutants
developing vs developed countries
developing
use more subsistence fuels such as wood, manure, charcoal (biomass)
often combusted indoors with poor ventilation, leading to high concentrations
about 3.5 - 4.3 million of 3 billion people who use subsistence fuels die annually
developed
use more commercial fuels (coal, oil, natural gas) supplied by utilities
typically burned in closed, well ventilated furnaces, stoves, etc.
many indoor air pollutants in developed nations come from chemicals in products
adhesives in furniture
cleaning supplies
insulation
lead paint
classes of indoor air pollutants
natural
radon-222
mold
dust
anthropogenic
VOCs
formaldehyde
lead
major indoor air pollutants in developed nations come from chemicals in products: adhesives in furniture, cleaning supplies, insulation, lead paint
indoor pollutants - description - effects
asbestos | long, silicate particle previously used in insulation | linked to lung cancer and asbestosis |
CO | produced by incomplete combustion (low O2 or temp leads to some of the fuel not being combusted) | asphyxiant → causes suffocation due to CO binding to hemoglobin in blood, displacing O2 (lethal to humans in high concentrations) |
VOCs |
| irritate eyes, lungs, and bronchioles |
radon |
| second leading cause of lung cancer |
dust and mold |
| respiratory irritant (black mold spores are especially harmful) |
lead | found in paint in old homes (epa banned lead paint in ‘78) and lead water pipes (flint water crisis) | damages central nervous system of children due to smaller size and still developing brain |
7.6 Reduction of Air Pollutants
general methods
regulatory practices
clean air act → allow epa to set acceptable levels for criteria air pollutants
cafe vehicle standards
corporate average fuel economy → standards requiring the entire united states “fleet” of vehicles to meet certain average fuel
emissions inspections (VA)
conservation methods and practices
drive less, walk, bike, bus, etc.
alternative fuels/energy
solar, wind, hydro
reducing vehicle emissions
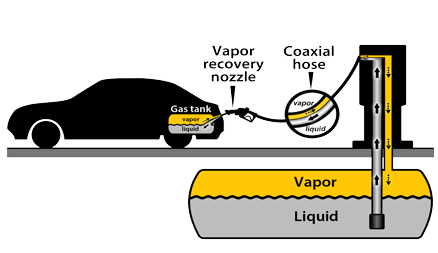
vapor recovery nozzle
capture hydrocarbon VOCs released from gasoline fumes during refueling
separate tube inside nozzle captures vapors and returns them to underground storage tank beneath the gas station
reduces VOCs
reduces benzene (carcinogen) released from gasoline vapors
catalytic converter (cc)
cc converts NOx, CO, and other hydrocarbons released by internal combustion engines into CO2, N2, O2, and H2O

scrubbers
dry scrubbers (NOx, SOx, VOCs)
large column/tube/pipe filled with chemicals that absorb or neutralize oxides (NOx, SOx, VOCs) from exhaust streams (emissions)
calcium oxide is a common dry scrubber additive which reacts with SO2 to form calcium sulfite
wet scrubbers (NOx, SOx, VOCs + PM)
may involve chemical agents that absorb or neutralize NOx, SOx, VOCs, but also include mist nozzles that trap PM in water droplets as well
mist droplets with pollutants and PM trapped in them fall to bottom of scrubber or get trapped at top by mist eliminator
sludge collection systems traps polluted water for disposal
electrostatic precipitator
power plant/factory emissions passed through device with a negatively charged electrode, giving particles a negative charge
negatively charged particles stick to positively charged collection plates, trapping them
plates discharged occasionally so particles fall down into collection hopper for disposal in landfills
7.7 Acid Rain
acid rain/deposition
NOx and SO2 are primary pollutants that cause most acid precipitation
NOx and SO2 react with O2 and H2O in the atm, forming nitric and sulfuric acid (secondary pollutants)
sulfuric and nitric acid dissociate in the presence of water into sulfate, nitrate, and hydrogen ions (H+)
acidic rain water (higher H+ concentration) decreases soil and water pH, limiting tree growth in forests
reducing NOx and SO2 emissions reduces acid deposition
higher cafe standards
more public transit
renewable energy sources
more efficient electricity use
acid rain has decreased significantly since the passage of the clean air act
effects of acid deposition
acidity = higher H+ ion concentration, lower pH
effects of acid deposition may be direct, such as a decrease in the pH of lake water, or indirect
often difficult to determine whether an effect is direct or indirect
greatest effect of acid deposition is on aquatic ecosystems
soil pH can also drop
corrosion of man-made structures
indicator species can be surveyed and used to determine conditions of an ecosystem (soil, water, etc.)
ex) high white moss/filamentous algae population indicated pH < 6.0
high crustacean population indicates pH > 6.0
mitigating acid rain
limestone
calcium carbonate (CaCO3) reacts with H+ ions, forming HCO3 and giving off Ca2+
this “neutralizes” acidic water/soil, moving it closer to a pH of 7
regions with limestone bedrock have some natural buffering of acid rain
humans can also add crushed limestone to soils/waters to neutralize
7.8 Noise Pollution
sources of noise pollution
any noise at great enough volume to cause physiological stress (difficulty communicating, headaches, confusion) or hearing loss
construction
ex) jack hammers, trucks, concrete pouring
transportation
ex) cars, busses, trains
industrial activity
ex) manufacturing plants
domestic activity
ex) neighbor’s music, lawn mowing, home projects
effects of noise pollution
noise pollution can disrupt animal communication, migration, and damage hearing
effects on wildlife (land)
causes physiological stress
ex) caterpillar hearts beat faster when exposed to simulated highway noise pollution → could drive pollinator species to decline
masks natural sounds
ex) mating calls, prey/predator noises
effects on aquatic organisms
aquatic noise pollution comes from the noise of ship engines, military sonar, and seismic air blasts from oil and gas surveying ships
causes physiological stress
ex) hearing loss, disrupted communication, mating calls, predator/prey navigation
disrupts migration (especially whales, as communication underwater can be disrupted)