MYP Biology 9 HL Unit 2
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
30 Terms
Covalent Bond
Occurs when electrons are shared between two atoms
Isotope
Atoms of the same element that have same numbers of protons but different numbers of neutrons
Surface Tension
Caused by cohesion, allows the uppurmost molecules to create a type of thin surface
Duet Rule
Hydrogen and Helium need 2 electrons in their outermost shell to become stable
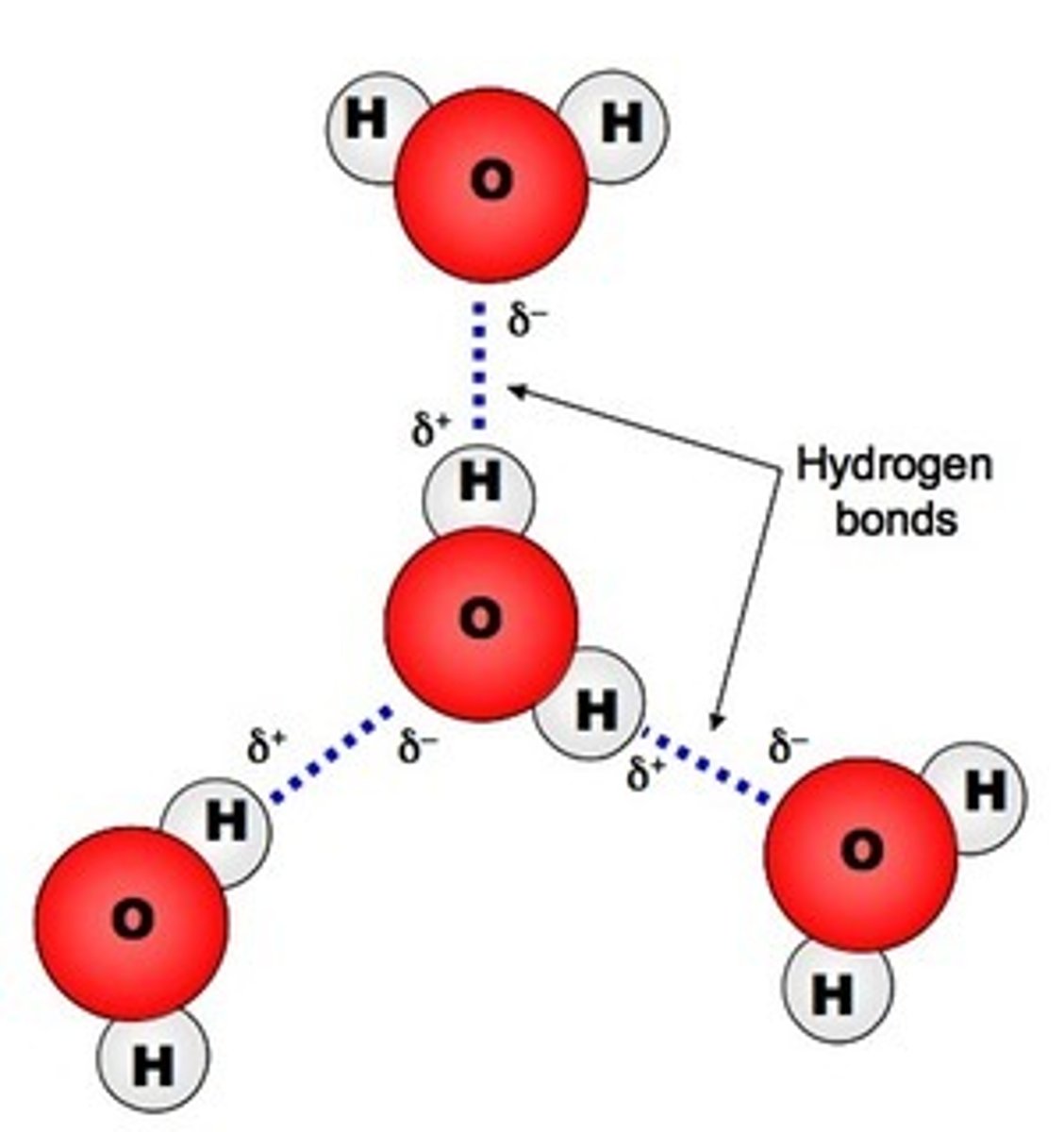
5. Draw and label the molecules and partial charges of a water molecule. Also be able to correctly place hydrogen bonds.
Compare drawing to image --->
Anion
Negatively charged ion
Atomic number
Number of protons
Heat Capacity (water)
Water can absorb a high amount of heat energy with only a small change in temperature
Hydrogen Bond
Forms between molecules that contain a hydrogen atom in a polar covalent bond. Hydrogen atom will have a slightly positive charge.
Matter
Anything that has mass and takes up space
List the 7 properties of water
Cohesion, adhesion, universal solvent, polarity, heat capacity, and surface tension, and hydrogen bonding
Adhesion
Sticks to other charged molecules
Cation
Positively charged ion
Cohesion
Sticks to itself
Electron
Negatively charged subatomic particle, revolving around an atom Mass 0 amu
Element
A substance that cannot be broken down into simpler substances
Ion
A charged atom
Ionic Bond
Electrons are transferred from one atom to another. One atom loses electrons, and one atom gains
Mass number
sum of the number of protons and neutrons
Nucleus
Center of an atom
Proton
Positively charged subatomic particle in the nucleus of an atom. Mass 1 amu
Universal solvent
Ability to dissolve many different solutes
Mass
The amount of matter in an object
List the 4 elements most common in living organisms.
Carbon, Hydrogen, Oxygen, Nitrogen (CHON)
Explain why most atoms tend to interact with other atoms. Explain in terms of their outermost electrons.
Atoms need to fill up their outermost shell (8 vElectrons)to become stable, so they bond with other atoms and share electrons so that they become stable
Describe the octet rule.
An atom can gain, lose, or share electrons to obtain 8 electrons in their outermost shell.
Define polarity.
Atoms share electrons unevenly because some atoms attract more strongly than others
Why is water a polar molecule?
Water is a polar molecule because it does not share its electrons equally.
Which partial charges are on the hydrogen atoms in water?
Partial positive charges.
Which partial charge is on the oxygen atom in water?
Partial negative charge.