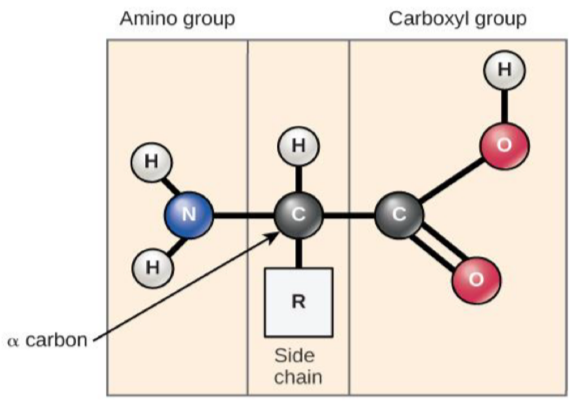
Amino acids and primary structure
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
Amino acids basic facts
Monomer units of proteins (primary structure)
Each AA has a name, normally ending in -ine.
For convenience, these are often abbreviated E.g. Gly for Glycine
Essential AA= cannot be made in the body, must be consumed
Non-essential AA= cannot be made in the body
Electronegativity values and bond type
if electronegativity values are greater than 1.7 = more ionic
If values less than 1.7= more likely covalent
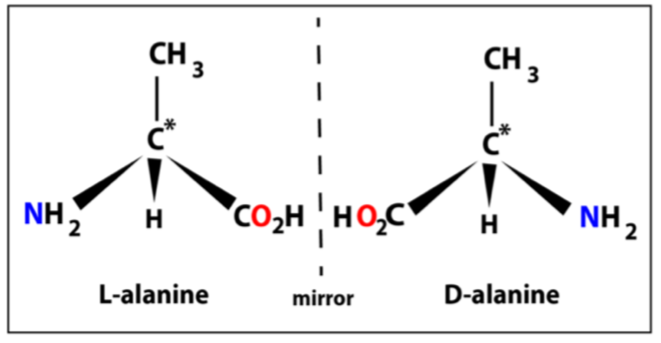
Chirality of amino acids
The amino (-NH2) substituents is the “main” one
When its on the left = L configuration
When on the right = D configuration
Other 3 are: H, CH3 and CO2H
4 sub-groups of amino acids
1) Hydrophobic AA with non-polar R groups
Glycine- no chirality as R group = H
Proline- found in rigid proteins e.g. collagen
2) Positively charged AA
Histidine- found in active sites
Has an imidazole ring= binds and releases H+
3) Polar AA
e.g.Cysteine, contains an SH group
Can covalently bond with another Cysteine/itself
4) Negatively charged AA
e.g. Glutamate, excitatory NT
What are pH and pKa?
PH = H+ concentration
most bodily fluids 6.5-8pH
Dissociation constant = Ka (Inconvenient)
PKa is a more manageable number, allows for simple comparisons etc.
AB— A+ and B-
How readily an acid donates a proton
Smaller pKa values = stronger acidity e.g. lactic acid = 3.8
AA can exist in various protonated states = different pKas
If the pH of a solution is the same as the pKa of the AA, 50:50 protonated : deprotonated.
If the pH is lower than pKa = protonated, and pH higher = deprotonated.
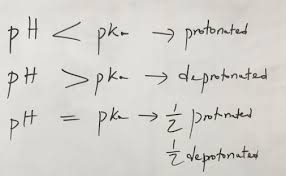
How to work out Ka and pKa
Ka = (A+)(B-)/AB.
Where A and B are ions that combine to form an acid.
PKa= -log10Ka
Index of acidity of acids
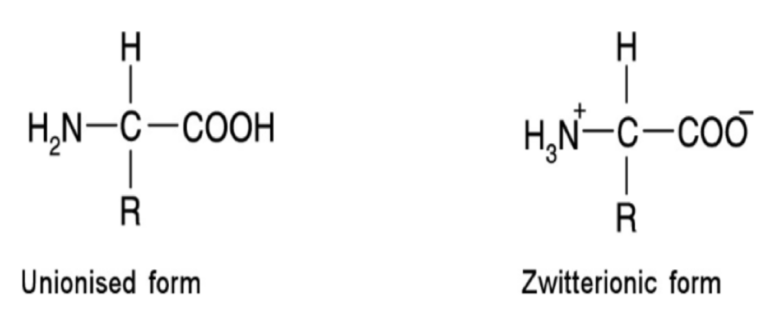
What are zwitterions?
Molecules that posses both +ve and -ve charges, so have an overall neutral charge
Amino acids in a neutral pH solution exist predominantly as dipolar ions (zwitterions)
Can exist as either unionised or zwitterionic
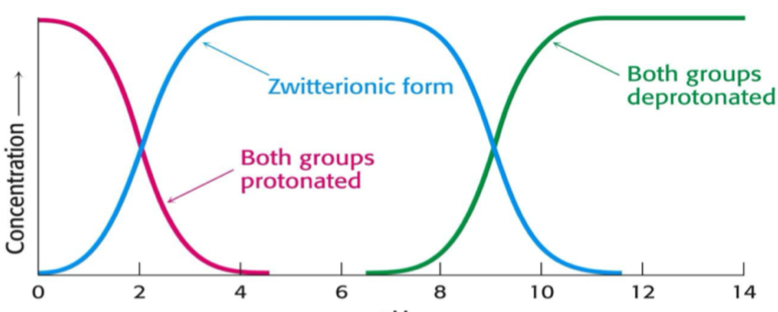
How does the protonation of a molecule change with pH?
At low pH, the concentration of both groups being protonated is highest.
At middle (neutral) pH, the zwitterionic form is most common
At high pHs deprotonated forms are most common
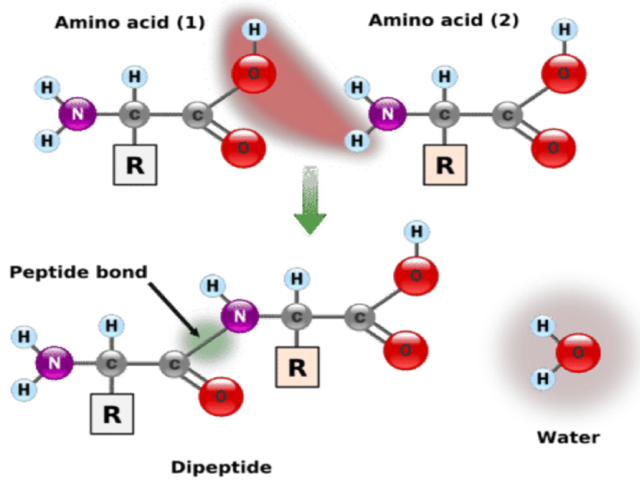
Peptide bond formation and structure
Condensation reaction
A water molecule is released during the formation of a peptide bond as an amine group joins to a carboxylate group (dehydration synthesis)
The amino end is the beginning of the chain.
Main chain and side chains vary with molecules
Backbone = rich in H bonding potential
Each AA contains a CO double bond = good hydrogen bond acceptor OR NH group = H bonding potential H donor
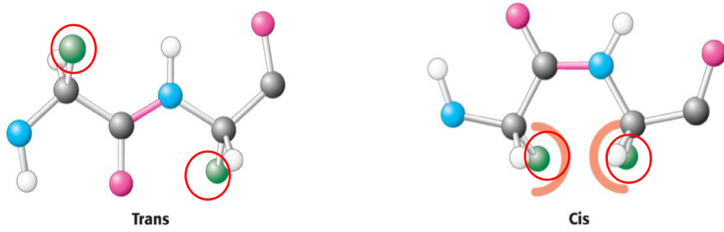
Arrangement of a peptide bond
Peptide bonds are planar around the CO group of one AA and the NH group of the other. Typical bond length between = 1.32 Å
Trans-peptide bonds = alpha C’s on opposite sides
Cis-peptide bond = alpha C’s on same side
Almost all are Trans- as it avoids steric clashes (atoms become too close to eachother)