C2.5 Simple molecules and covalent bonds
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
What is a covalent bond?
Bond formed when a pair of electrons is shared between 2 non-metals atoms leading to noble gas electronic configurations
Explain in terms of structure and bonding the properties of simple molecular compounds
Low melting points and boiling points: weak electrostatic forces of attraction between the molecules which require very little energy to break
Poor electrical conductivity: don’t have any free ions or electrons that can carry charge, most covalent compounds act as insulators when they are solid
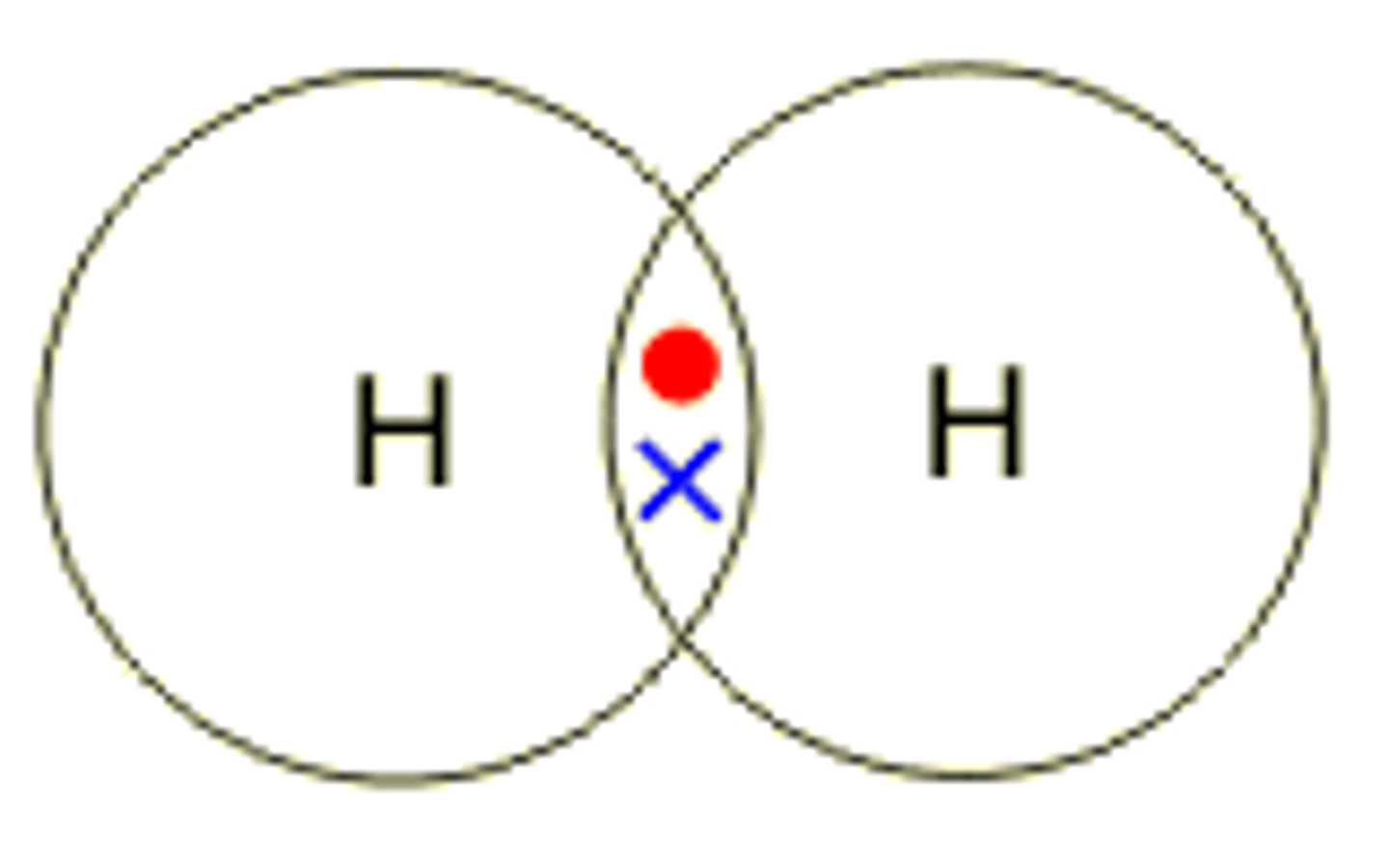
Draw the covalent bond for hydrogen
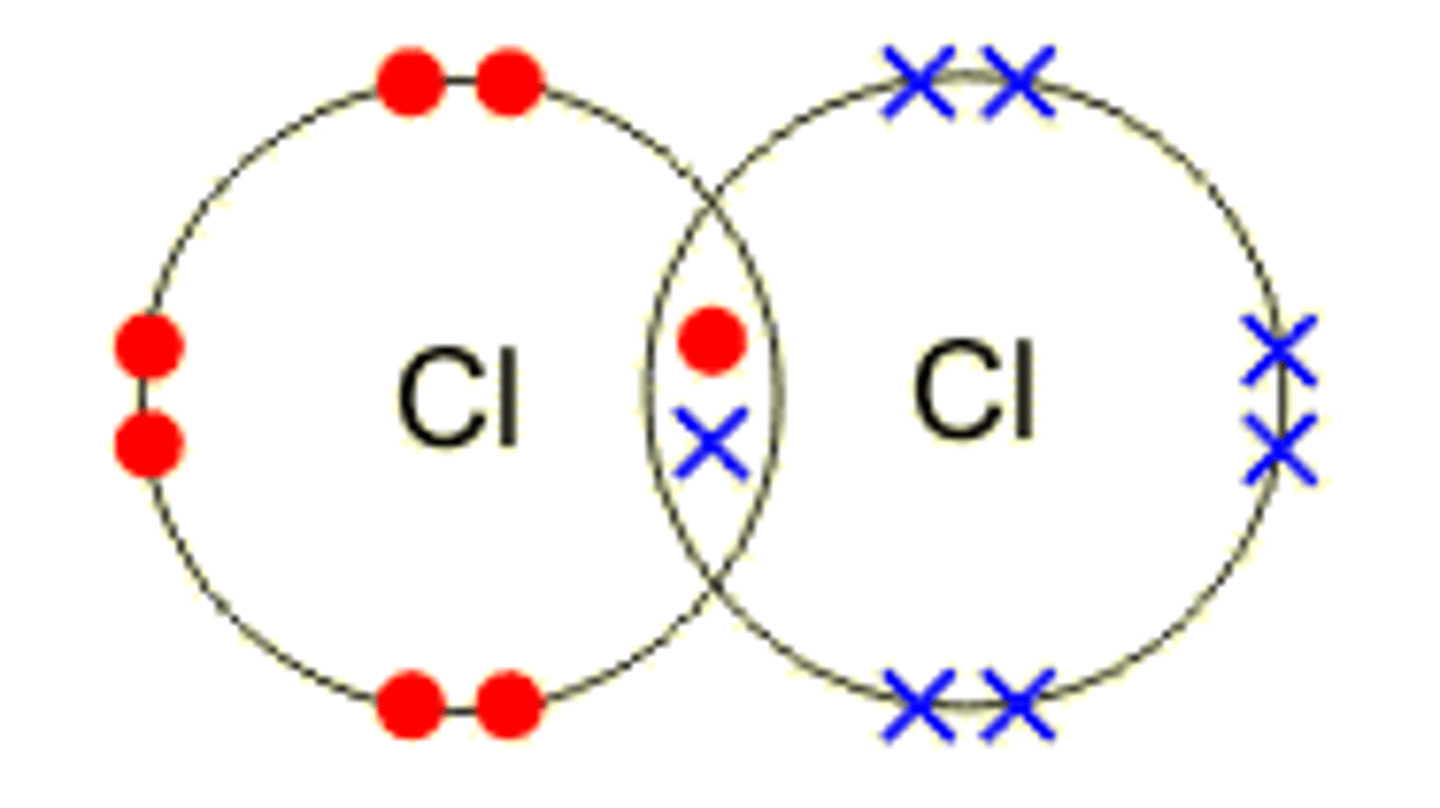
Draw the covalent bond for chlorine
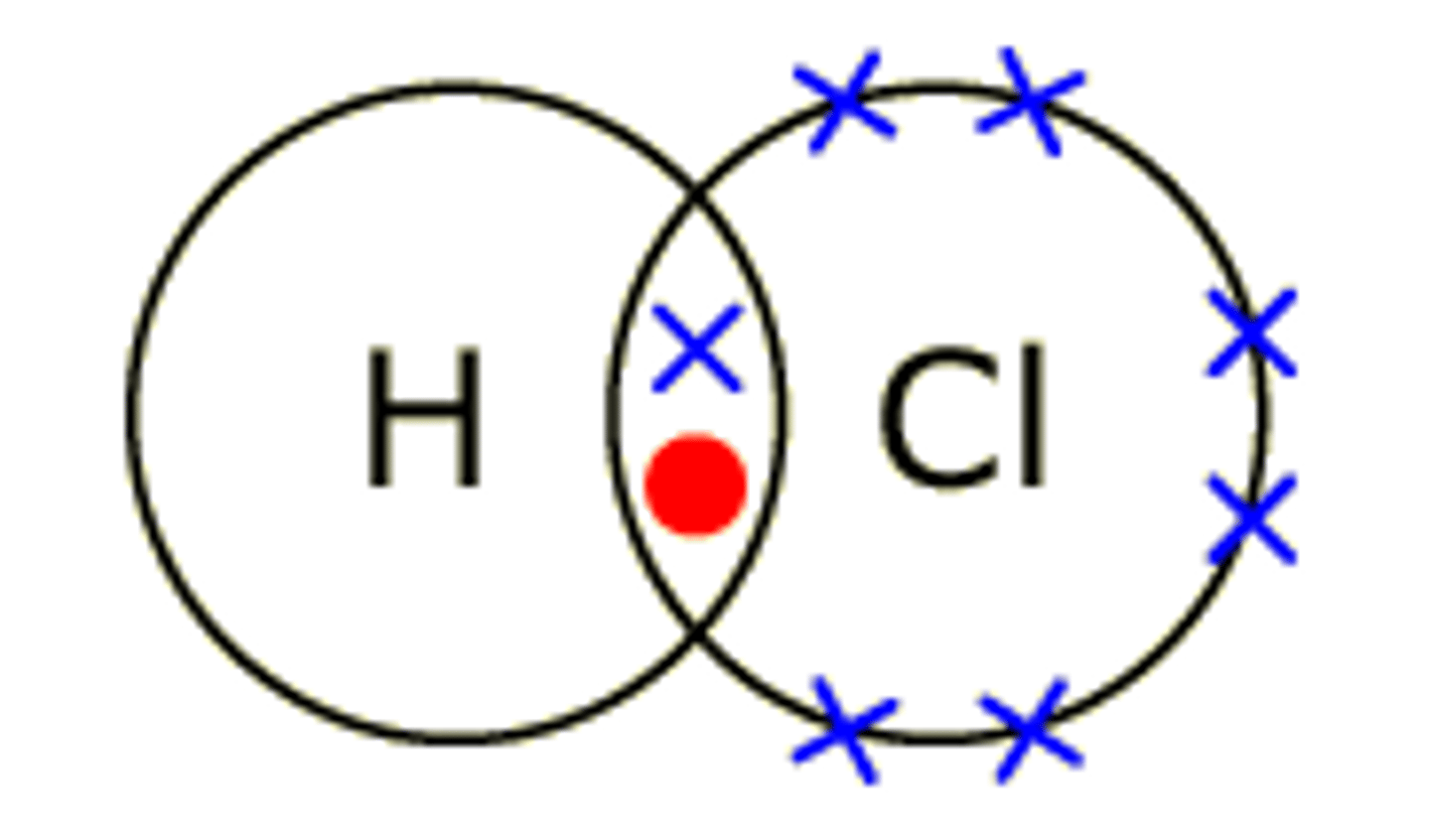
Draw the covalent bond for hydrogen chloride
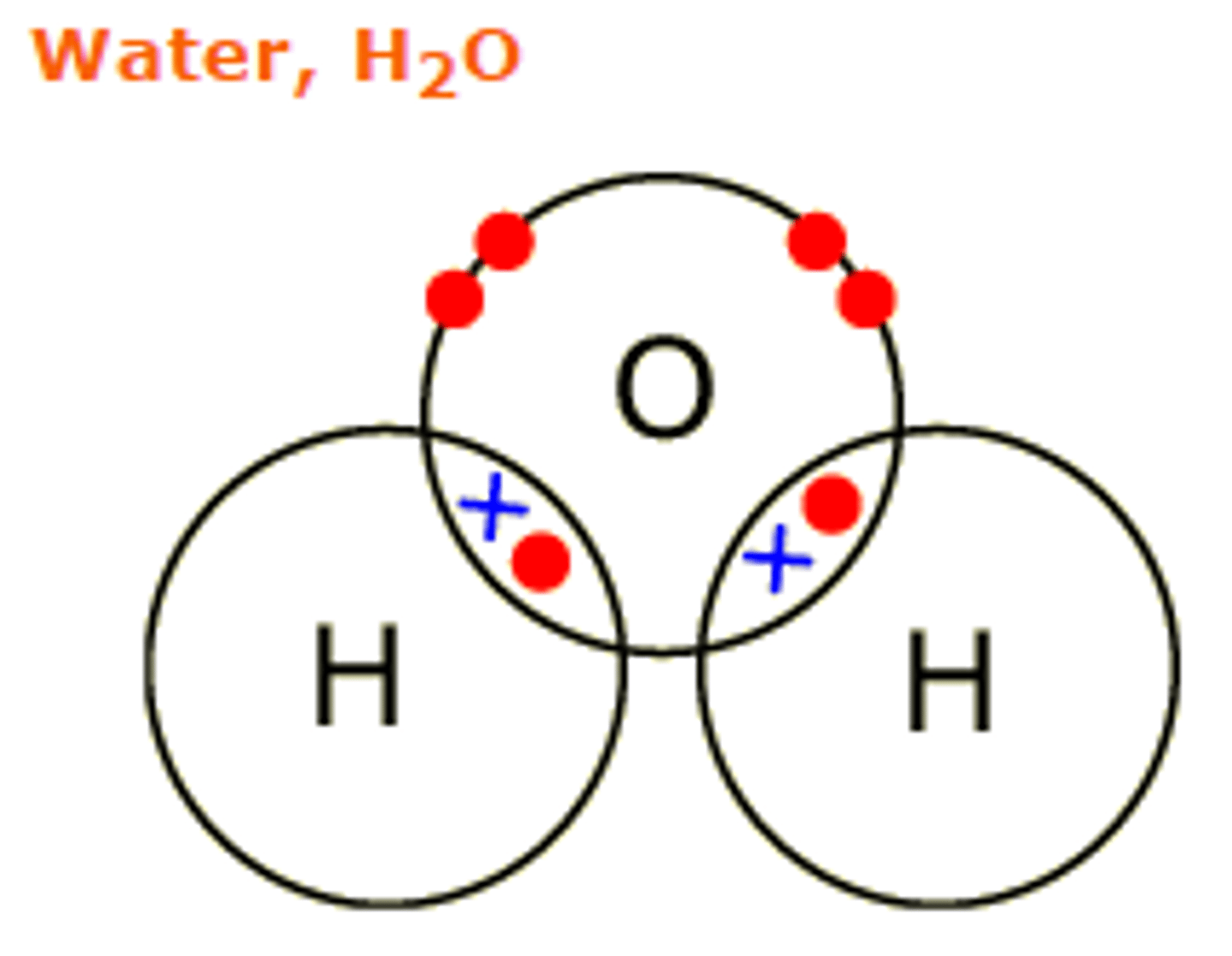
Draw the covalent bond for water
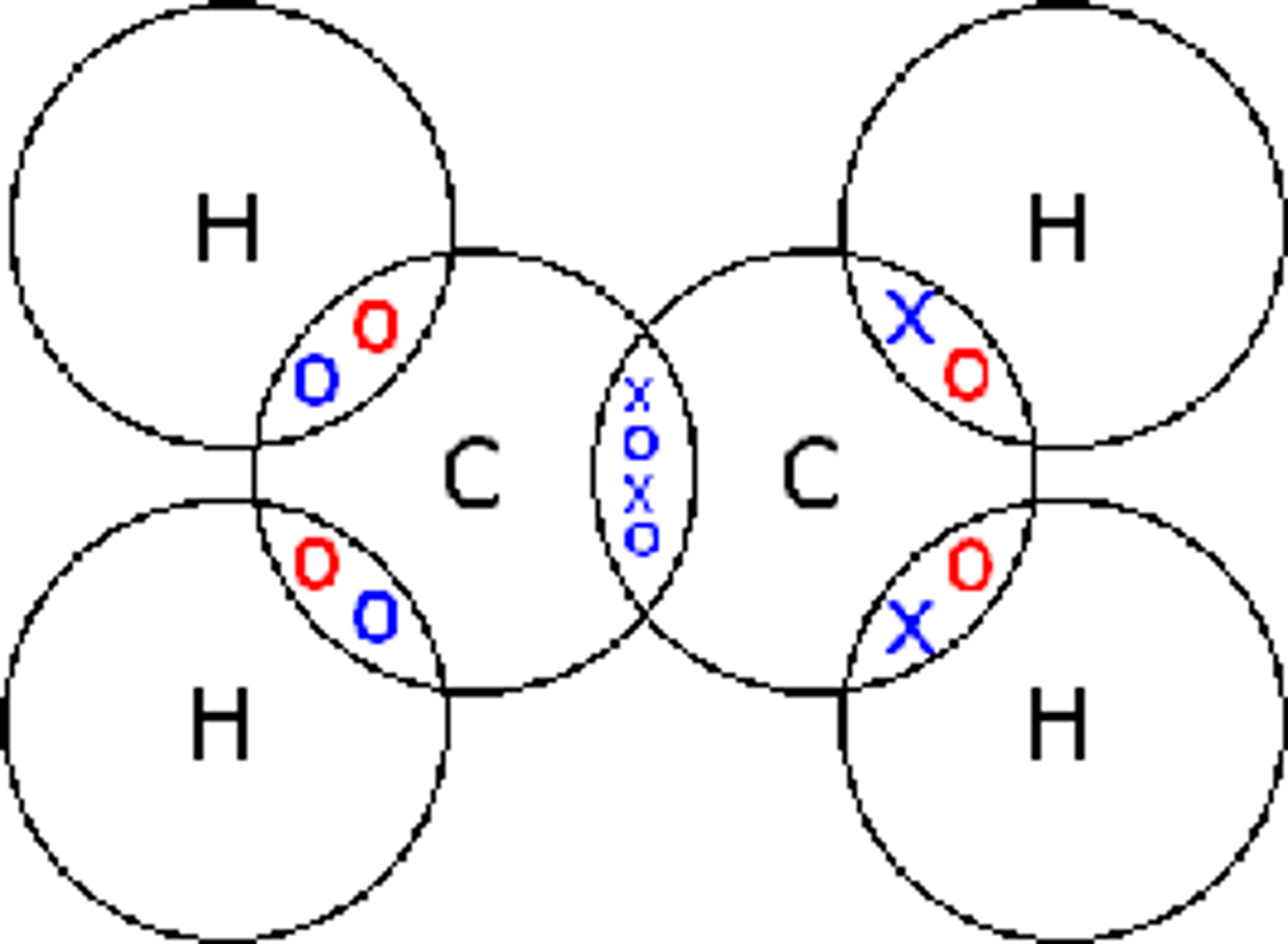
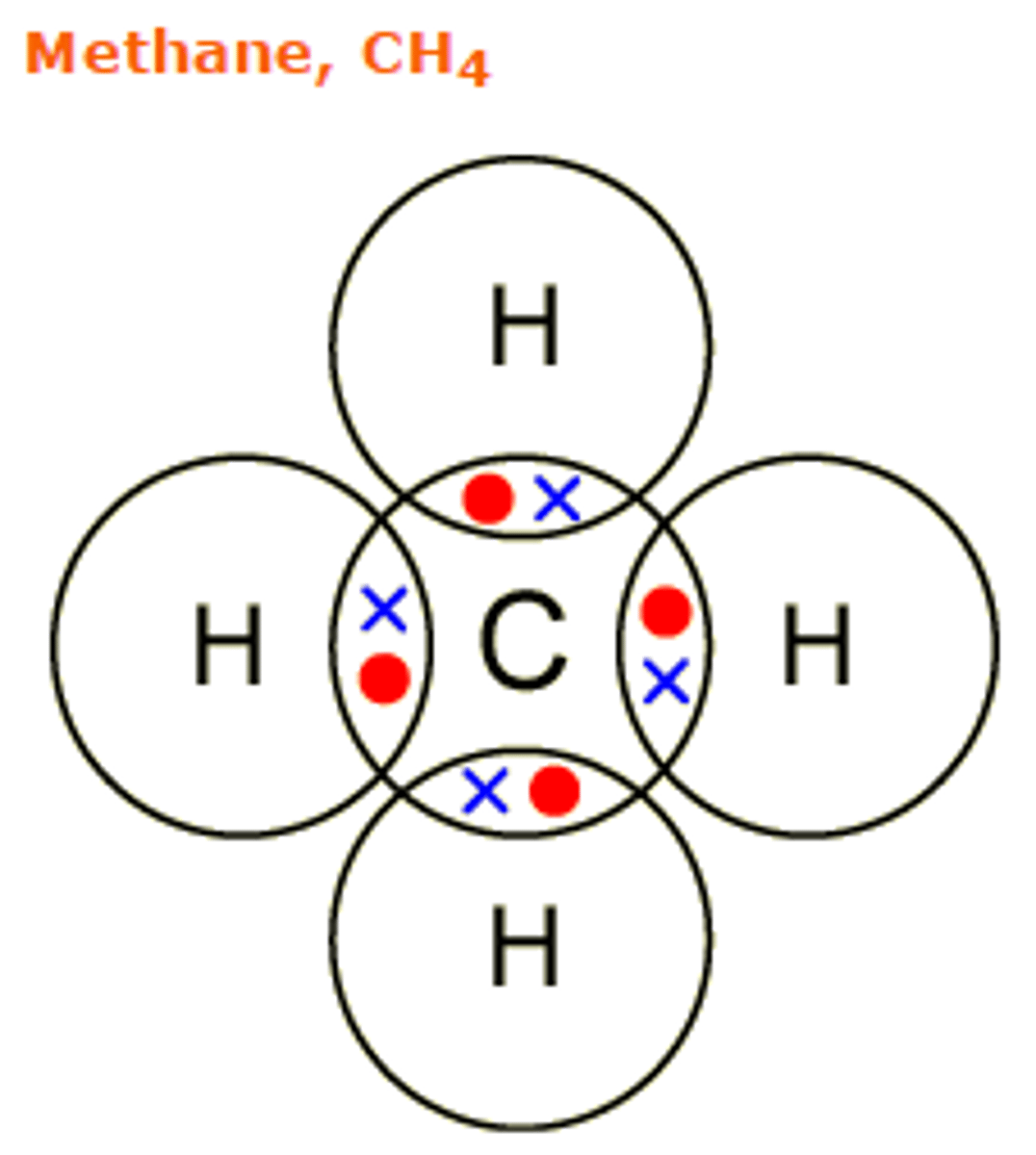
Draw the covalent bond for methane
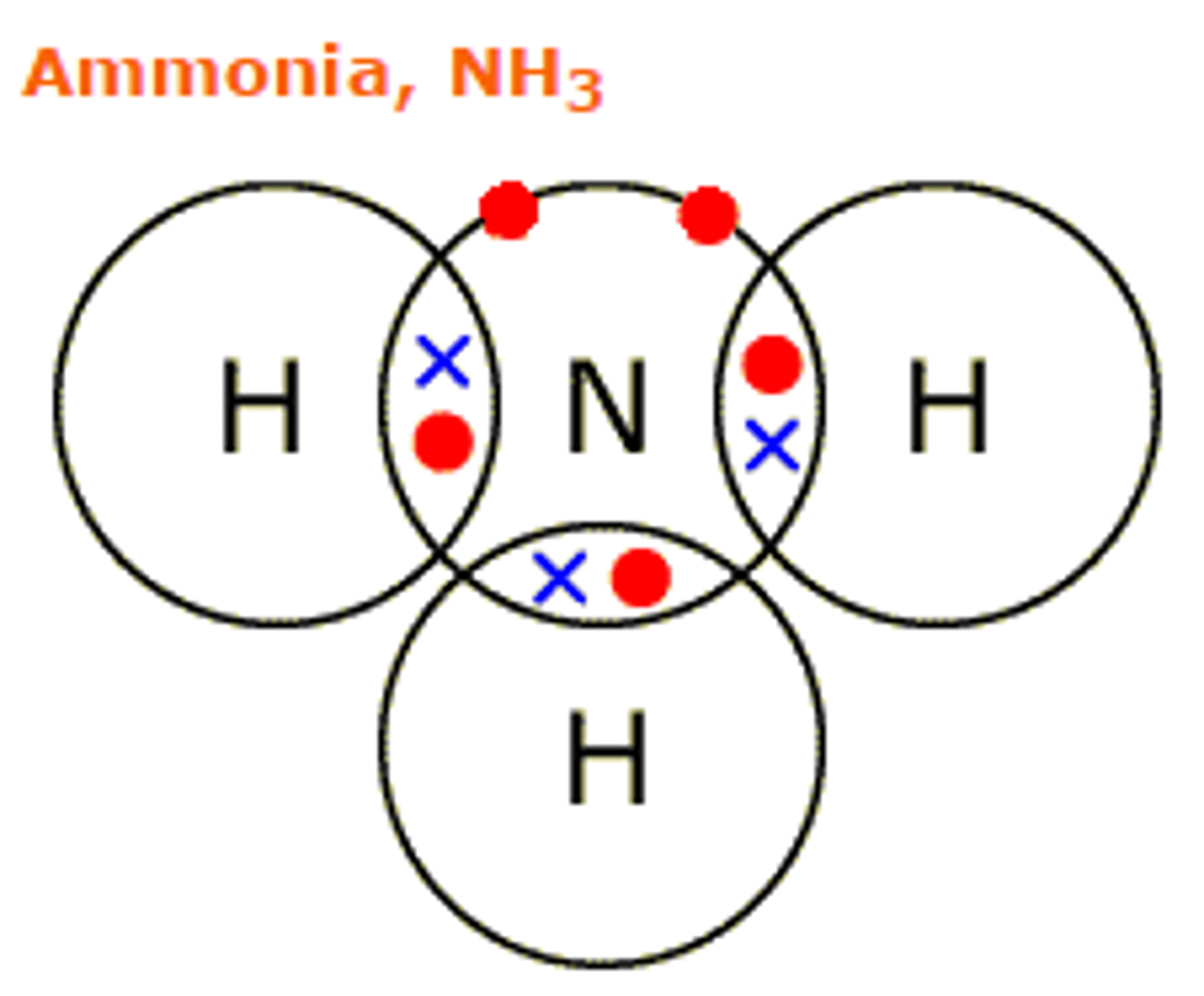
Draw the covalent bond for ammonia
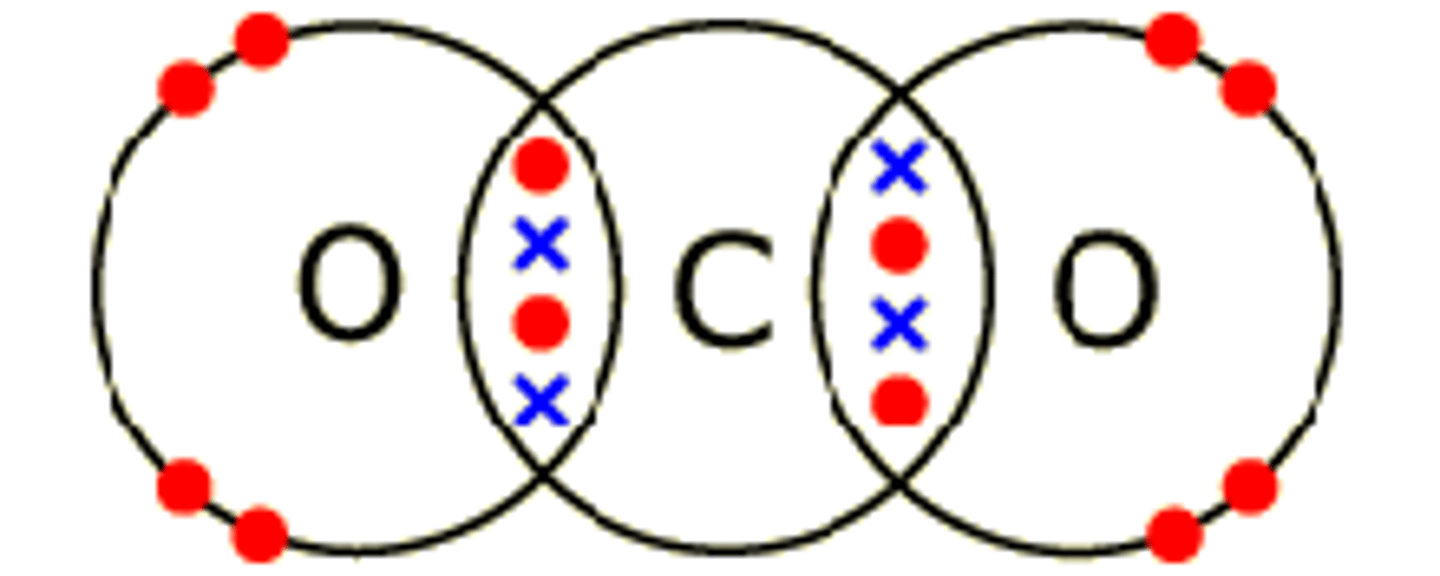
Draw the covalent bond for carbon dioxide
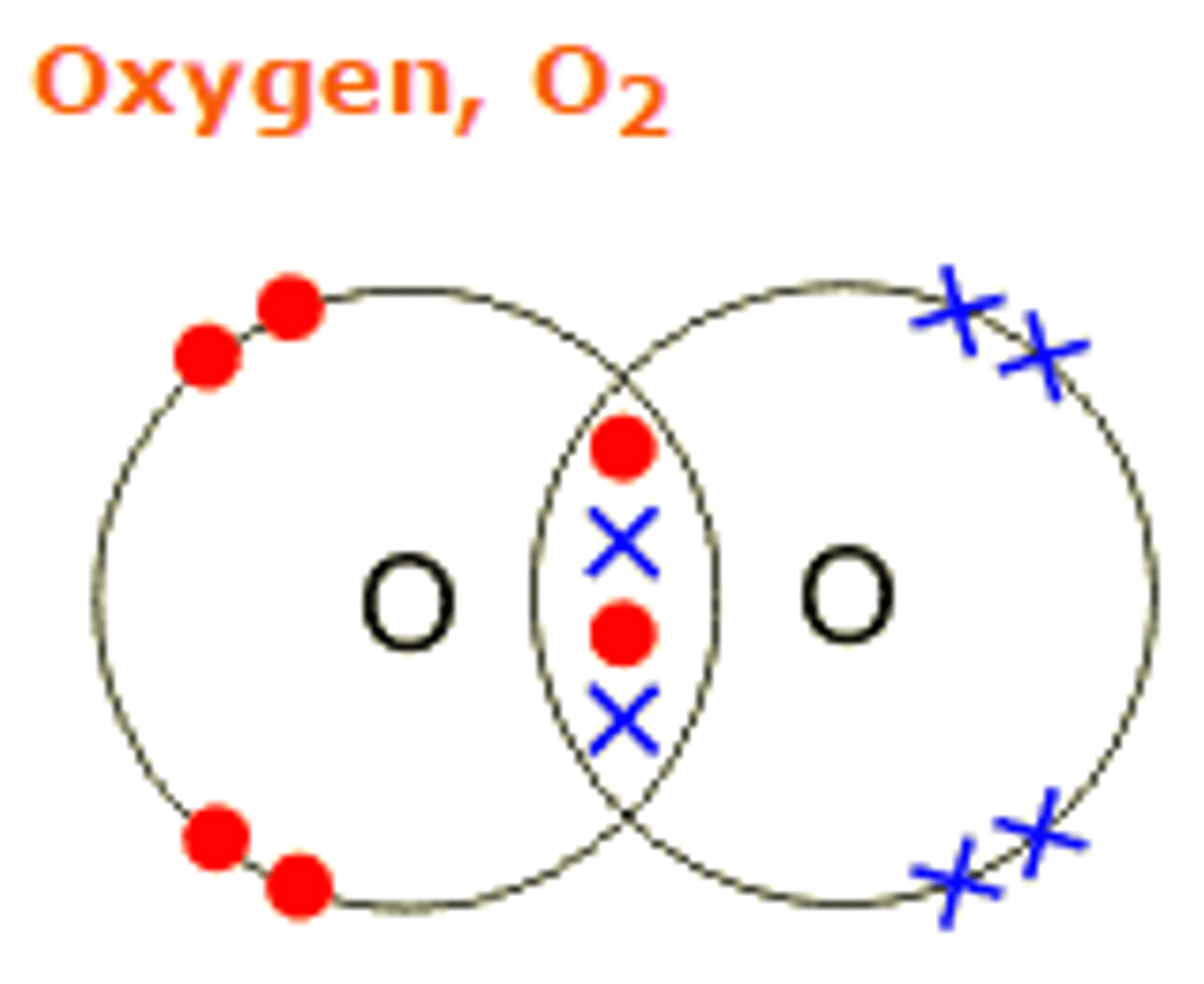
Draw the covalent bond for oxygen
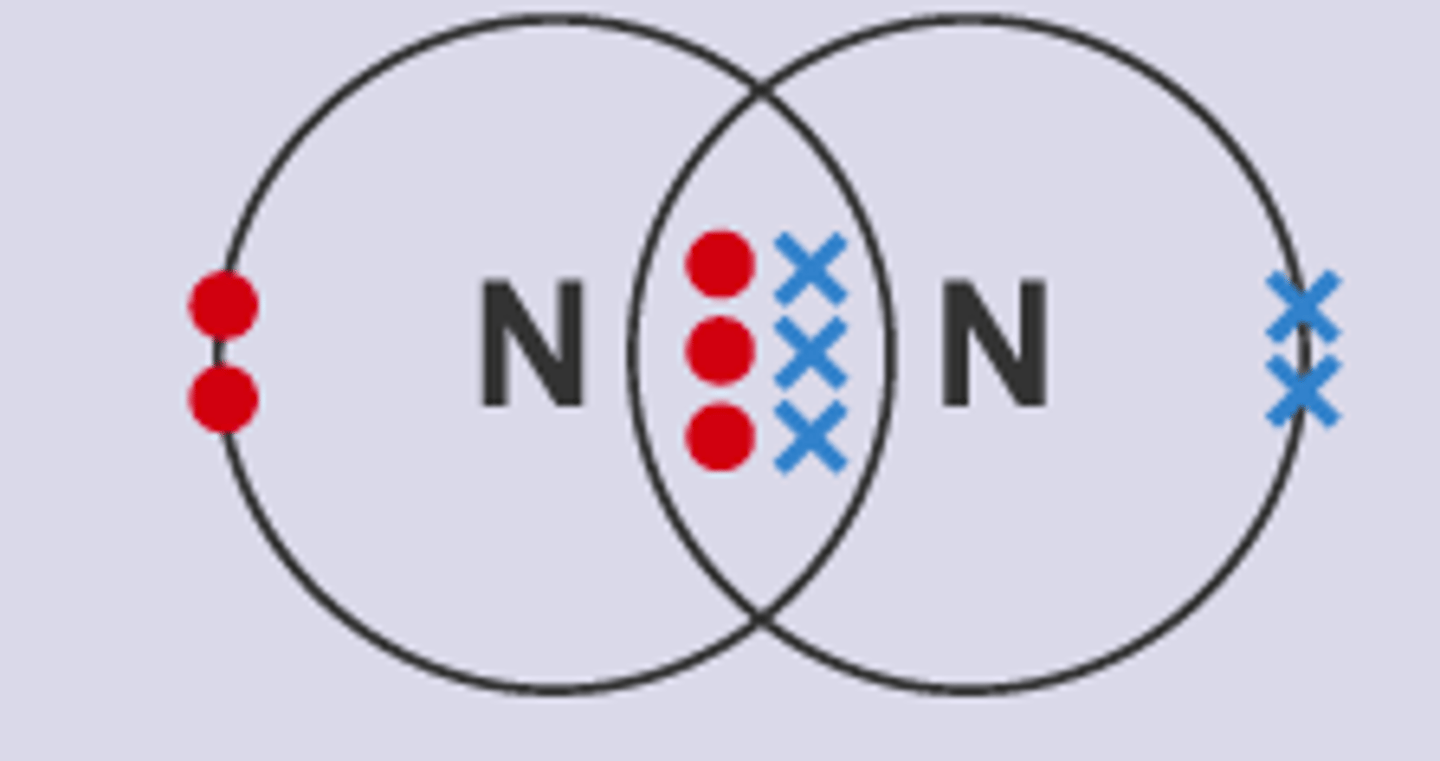
Draw the covalent bond for nitrogen
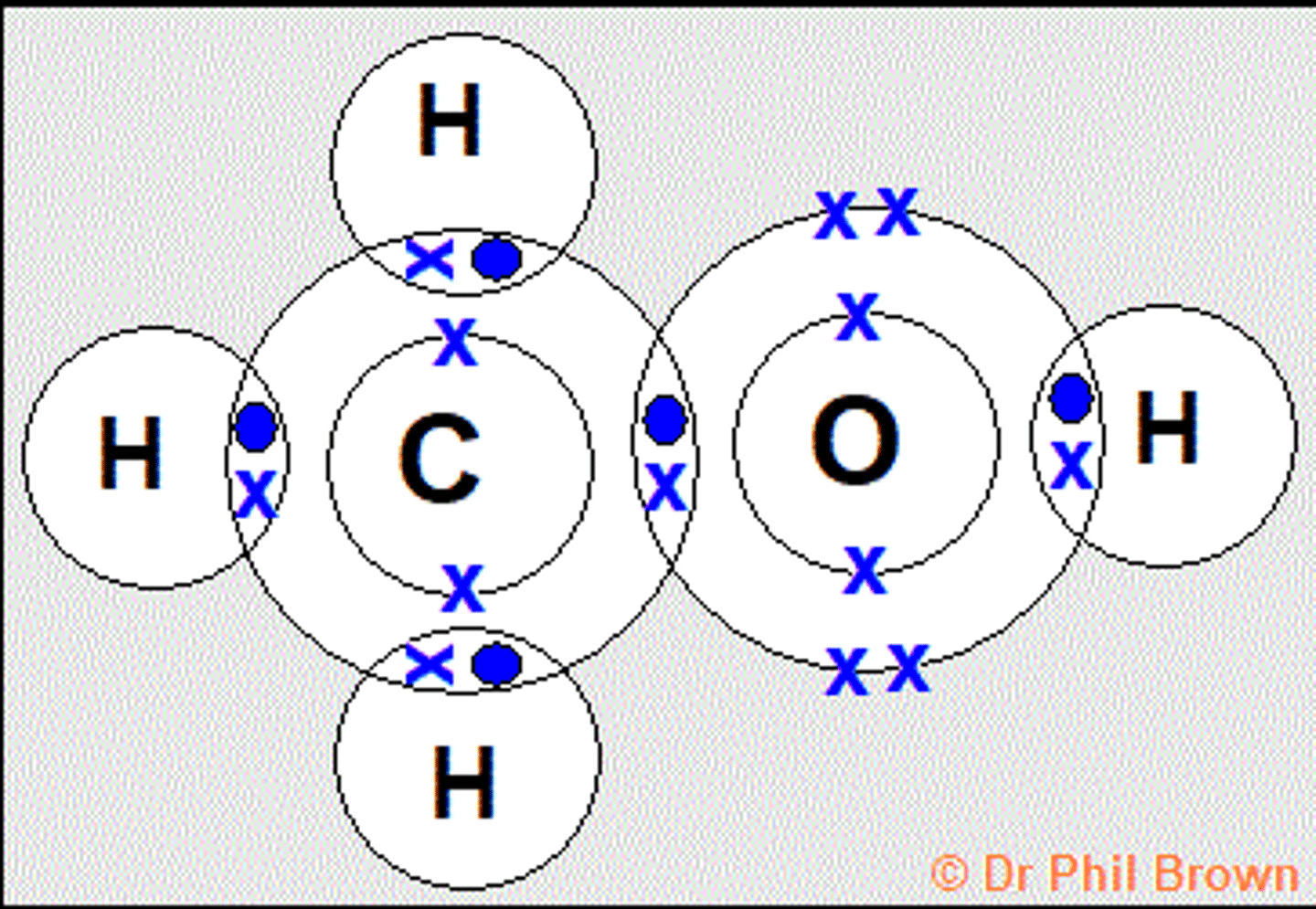
Draw the covalent bond for methanol
Ch3OH
Draw the covalent bond for ethene
C2H4